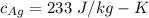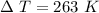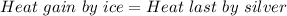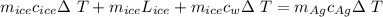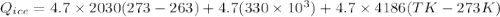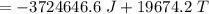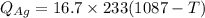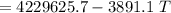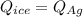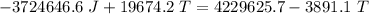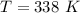A 4.7-kg block of ice originally at 263 K is placed in thermal contact with a 16.2-kg block of silver (cAg = 233 J/kg-K) which is initially at 1067 K. The H2O - silver system is insulated so no heat flows into or out of it. At what temperature will the system achieve equilibrium?

Answers: 3


Another question on History

History, 21.06.2019 15:10
Which of the following statements is true for post-processual archaeology? select one: a. its research by inductive reasoning focuses on the major events in the history of humankind. b. its research uses primarily scientific and anthropological methods and techniques. c. its research focuses on all individuals associated with the ancient remains.
Answers: 1

History, 22.06.2019 03:00
Synthesizing new ideas from a speaker with what one already knows about the subject to see if the information makes sense is an example of a.questioning and challenging b.identifying differences c. forming a statement d.seeing the boader context
Answers: 2

History, 22.06.2019 07:00
At the time of the brown v. board of education decision,schools were segregated everywhere in the country.several states had laws requiring segregated schools.schools in nearly every state has been desegregated.segregation was common in the north, but not in the south.
Answers: 1

History, 22.06.2019 15:00
How does smith characterize the colonizing effort and why does he so characterize the effort to settle jamestown? what was the most difficult challenge faced by the colonists who established jamestown? how was this most difficult challenge affected by other demands and challenges that confronted the first english to establish a permanent settlement in north america?
Answers: 2
You know the right answer?
A 4.7-kg block of ice originally at 263 K is placed in thermal contact with a 16.2-kg block of silve...
Questions



History, 05.02.2021 19:20




Mathematics, 05.02.2021 19:20


History, 05.02.2021 19:20


English, 05.02.2021 19:20




Mathematics, 05.02.2021 19:20


Mathematics, 05.02.2021 19:20

Mathematics, 05.02.2021 19:20