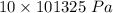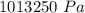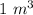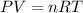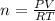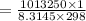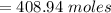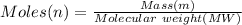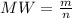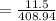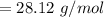Engineering, 28.06.2021 14:00 oliviaclerk5
In order to fill a tank of 1000 liter volume to a pressure of 10 atm at 298K, an 11.5Kg of the gas is required. How many moles of the gas are present in the tank? What is the molecular weight of the gas? Assuming that the gas to be a pure element can you identify it?

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 18:10
Fluids at rest possess no flow energy. a)- true b)- false
Answers: 3

Engineering, 04.07.2019 18:10
Afluid flows with a velocity field given by v=(x/t)i.. determine the local and convective accelerations when x=3 and t=1.
Answers: 2

Engineering, 04.07.2019 18:10
At 12 noon, the count in a bacteria culture was 400; at 4: 00 pm the count was 1200 let p(t) denote the bacteria cou population growth law. find: (a) an expression for the bacteria count at any time t (b) the bacteria count at 10 am. (c) the time required for the bacteria count to reach 1800.
Answers: 1

Engineering, 04.07.2019 18:10
A-mn has a cubic structure with a0 0.8931 nm and a density of 7.47 g/cm3. b-mn has a different cubic structure, with a0 0.6326 nm and a density of 7.26 g/cm3. the atomic weight of manganese is 54.938 g/mol and the atomic radius is 0.112 nm. determine the percent volume change that would occur if a-mn transforms to b-mn.
Answers: 2
You know the right answer?
In order to fill a tank of 1000 liter volume to a pressure of 10 atm at 298K, an 11.5Kg of the gas i...
Questions


Biology, 02.05.2021 05:50


Biology, 02.05.2021 05:50

Mathematics, 02.05.2021 05:50



Mathematics, 02.05.2021 05:50


History, 02.05.2021 05:50


Mathematics, 02.05.2021 05:50

Mathematics, 02.05.2021 05:50


Mathematics, 02.05.2021 05:50

Physics, 02.05.2021 05:50



Mathematics, 02.05.2021 05:50

Mathematics, 02.05.2021 05:50