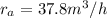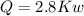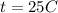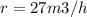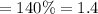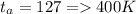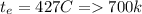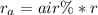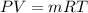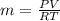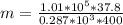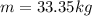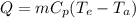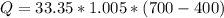Engineering, 14.06.2021 22:10 spiritedawayoy6378
Methane gas (CH4) at 25oC, 1 atm, and a volumetric flow rate of 27m3/h enters a furnace operating at steady-state. The methane burns completely with 140% of theoretical air that enters at 127oC, 1 atm. Products of combustion exit at 427oC, 1 atm. Determine:
(a) the volumetric flow rate of the air, in m3/h.
(b) the rate of heat transfer from the furnace, in kJ/h.

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 18:10
What difference(s) did you notice using a pneumatic circuit over hydraulic circuit.explain why the pneumatic piston stumbles when it hits an obstacle.
Answers: 2

Engineering, 04.07.2019 18:10
Acompressor receives the shaft work to decrease the pressure of the fluid. a)- true b)- false
Answers: 3

Engineering, 04.07.2019 18:20
Asolid cylinder is concentric with a straight pipe. the cylinder is 0.5 m long and has an outside diameter of 8 cm. the pipe has an inside diameter of 8.5 cm. the annulus between the cylinder ad the pipe contains stationary oil. the oil has a specific gravity of 0.92 and a kinematic viscosity of 5.57 x 10-4 m2/s. most nearly, what is the force needed to move the cylinder along the pipe at a constant velocity of 1 m/s?
Answers: 3

Engineering, 04.07.2019 19:10
Asteam is contained in a rigid tank with a volume of 1 m3. initially, the pressure and temperature are 7 bar and 500 oc, respectively. the temperature drops due to cooling process. determine: (1) the temperature at which condensation begins in °c, (2) the fraction of the total mass that has condensed when the pressure decreased to 0.5 bar. (3) the volume in m3 occupied by saturated liquid at the final state?
Answers: 3
You know the right answer?
Methane gas (CH4) at 25oC, 1 atm, and a volumetric flow rate of 27m3/h enters a furnace operating at...
Questions


Mathematics, 27.08.2019 23:00

Mathematics, 27.08.2019 23:00

Mathematics, 27.08.2019 23:00



Mathematics, 27.08.2019 23:00

Social Studies, 27.08.2019 23:00


Biology, 27.08.2019 23:00



Mathematics, 27.08.2019 23:00


Mathematics, 27.08.2019 23:00


History, 27.08.2019 23:00


Mathematics, 27.08.2019 23:00