Engineering, 08.03.2021 19:10 ahoney2233
Initially, 1 lbm of water is at rest at 14.7 psia and 70 F. The water then undergoes a process where the final state is 30 psia and 700 F with a velocity of 100 ft/s and an elevation of 100 ft above the starting location. Determine the increases in internal energy, potential energy, and kinetic energy of the water in Btu/lbm. Compare the increases in potential energy and kinetic energy individually to the change in the internal energy.

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 03:10
What precautions should you take to prevent injuries when dealing with heavy loads?
Answers: 1

Engineering, 04.07.2019 18:10
If a particle moves along a path such that r : (3 sin t) m and ? : 2t rad, where t is in seconds. what is the particle's acceleration in m/s in 4 seconds? a)- 16.43 b)- 16.29 c)- 15.21 d)- 13.79
Answers: 1

Engineering, 04.07.2019 18:10
Apump is used to circulate hot water in a home heating system. water enters the well-insulated pump operating at steady state at a rate of 0.42 gal/min. the inlet pressure and temperature are 14.7 lbf/in.2, and 180°f, respectively; at the exit the pressure is 60 lbf/in.2 the pump requires 1/15 hp of power input. water can be modeled as an incompressible substance with constant density of 60.58 lb/ft3 and constant specific heat of 1 btu/lb or. neglecting kinetic and potential energy effects, determine the temperature change, in °r, as the water flows through the pump.
Answers: 1

Engineering, 04.07.2019 18:20
Prove the equivalence between the two statements of the 2nd law of thermodynamics (i.e., a violation of one statement leads to the violatio the other statement)
Answers: 2
You know the right answer?
Initially, 1 lbm of water is at rest at 14.7 psia and 70 F. The water then undergoes a process where...
Questions

Social Studies, 08.02.2021 23:00


Mathematics, 08.02.2021 23:00

Mathematics, 08.02.2021 23:00


Mathematics, 08.02.2021 23:00

Mathematics, 08.02.2021 23:00









Geography, 08.02.2021 23:00


Mathematics, 08.02.2021 23:00

Mathematics, 08.02.2021 23:00

Computers and Technology, 08.02.2021 23:00

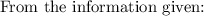
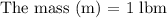

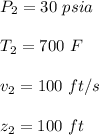
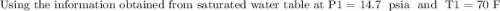
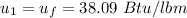
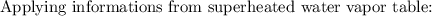

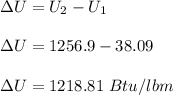

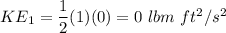


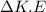 =
=