
Engineering, 21.12.2020 23:20 ella3714
The van der Waals equation is a modification of the ideal gas equation. What two factors does this equation account for? A. (1) Real gas molecules exert forces on each other. (2) Gas molecules have energy. B. (1) Real gas molecules exert ionic forces on each other. (2) Gas molecules have energy C. (1) Real gas molecules exert forces on each other. (2) Gas molecules have volume. D. (1) Real gas molecules exert ionic forces on each other. (2) Gas molecules have volume. E. None of the above have BOTH of the two factors accurately stated HINT: Check Van der Waals equation.

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 18:10
Aflywheel accelerates for 5 seconds at 2 rad/s2 from a speed of 20 rpm. determine the total number of revolutions of the flywheel during the period of its acceleration. a.5.65 b.8.43 c. 723 d.6.86
Answers: 2

Engineering, 04.07.2019 18:10
The filament of an incandescent lamp has a temperature of 2000k. calculate the fraction of radiation emitted in the visible light band if the filament is approximated as blackbody
Answers: 2

Engineering, 04.07.2019 18:10
Which of the following refers to refers to how well the control system responds to sudden changes in the system. a)-transient regulation b)- distributed regulation c)-constant regulation d)-steady-state regulation
Answers: 1

Engineering, 04.07.2019 18:20
Derive the correction factor formula for conical nozzle i=-(1+ cosa) and calculate the nozzle angle correction factor for a nozzle whose divergence hal-fangle is 13 (hint: assume that all the mass flow originates at the apex of the cone.
Answers: 3
You know the right answer?
The van der Waals equation is a modification of the ideal gas equation. What two factors does this e...
Questions

Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50


History, 18.03.2021 02:50





History, 18.03.2021 02:50

Geography, 18.03.2021 02:50


Mathematics, 18.03.2021 02:50

Social Studies, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Biology, 18.03.2021 02:50



English, 18.03.2021 02:50


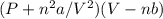 =nRT
=nRT

