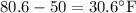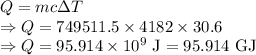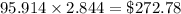Engineering, 18.12.2020 17:00 verawall39411
Thermodynamics fill in the blanks The swimming pool at the local YMCA holds roughly 749511.5 L (749511.5 kg) of water and is kept at a temperature of 80.6 °F year round using a natural gas heater. If you were to completely drain the pool and refill the pool with 50°F water, (blank) GJ (giga-Joules) of energy are required to to heat the water back to 80.6 °F. Note: The specific heat capacity of water is 4182 J/kg ⋅°C. The cost of natural gas per GJ is $2.844. It costs $ (blank) to heat the pool (to the nearest dollar).

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 18:10
Fluids at rest possess no flow energy. a)- true b)- false
Answers: 3

Engineering, 04.07.2019 18:10
The filament of an incandescent lamp has a temperature of 2000k. calculate the fraction of radiation emitted in the visible light band if the filament is approximated as blackbody
Answers: 2

Engineering, 04.07.2019 18:10
Manometers are good examples of measuring instruments, nowadays they are not as common as before. a)-capacitive probe gauges b)-gravitational gauges deformation ) gauges d)-digital gauges
Answers: 1

Engineering, 04.07.2019 18:10
Atmospheric air has a temperature (dry bulb) of 80° f and a wet bulb temperature of 60° f when the barometric pressure is 14.696 psia. determine the specific humidity, grains/lb dry air. a. 11.4 c. 55.8 d. 22.5 b. 44.1
Answers: 1
You know the right answer?
Thermodynamics fill in the blanks The swimming pool at the local YMCA holds roughly 749511.5 L (7495...
Questions

Business, 24.10.2021 03:20



English, 24.10.2021 03:20

English, 24.10.2021 03:20





History, 24.10.2021 03:20


Chemistry, 24.10.2021 03:20


History, 24.10.2021 03:20


SAT, 24.10.2021 03:20



Business, 24.10.2021 03:20

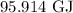
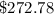
 = Change in temperature =
= Change in temperature =