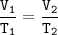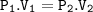Match each context to the type of the law that is most suitable for it.
Boyle’s Law
Cha...

Engineering, 30.10.2020 22:30 graymonky12
Match each context to the type of the law that is most suitable for it.
Boyle’s Law
Charles’ Law
Pascal’s Law
(Match them to the context it goes to)
At constant temperature, the volume of a confined gas is inversely proportional to the pressure to which it is subjected.
A change in pressure at an point in an enclosed fluid at rest is transmitted un diminished to all points in the fluid.
At constant pressure, the volume of a confined gas is directly proportional to the absolute temperature.

Answers: 1


Another question on Engineering

Engineering, 03.07.2019 15:10
Apiston-cylinder with a volume of 0.25 m3 holds 1 kg of air (r 0.287 k/kgk) at a temperature of 100 c. heat transfer to the cylinder causes an isothermal expansion of the piston until the volume triples. how much heat is added to the piston-cylinder?
Answers: 3

Engineering, 04.07.2019 18:10
Steel is coated with a thin layer of ceramic to protect against corrosion. what do you expect to happen to the coating when the temperature of the steel is increased significantly? explain.
Answers: 1

Engineering, 04.07.2019 18:20
Amixture of slurry and mud is to be pumped through a horizontal pipe of diameter 500 mm. the fluid behaves as a bingham plastic with a yield stress of 30 pa and viscosity 0.04 pa.s. describe the effects of the shear stress through a transverse section of the pipe by plotting the variation in shear stress and velocity profile: (i) just before the slurry starts to move (ii) as the slurry flows when the pressure gradient is double that in part (i)
Answers: 3

Engineering, 04.07.2019 19:10
The proportional limit is always greater than the yield strength for a material. a)-trune b)- false
Answers: 3
You know the right answer?
Questions

Social Studies, 27.08.2020 23:01

Mathematics, 27.08.2020 23:01





Social Studies, 27.08.2020 23:01




Mathematics, 27.08.2020 23:01

Computers and Technology, 27.08.2020 23:01


Mathematics, 27.08.2020 23:01



Advanced Placement (AP), 27.08.2020 23:01


Geography, 27.08.2020 23:01

History, 27.08.2020 23:01