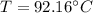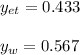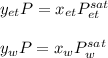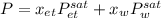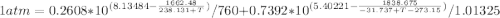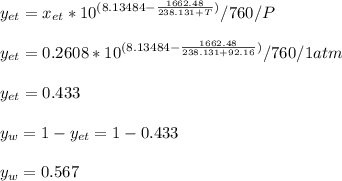Engineering, 02.09.2020 01:01 jordanrose98
Using Raoult’s law, estimate the boiling temperature and mole fractions in the vapor phase that is in equilibrium with a liquid having 0.2608 mol fraction of ethanol, in a mixture ethanol/water at P = 1 atm. If possible, solve non-numerically.

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 18:10
If a particle moves along a path such that r : (3 sin t) m and ? : 2t rad, where t is in seconds. what is the particle's acceleration in m/s in 4 seconds? a)- 16.43 b)- 16.29 c)- 15.21 d)- 13.79
Answers: 1

Engineering, 04.07.2019 18:10
Determine whether or not it is possible to compress air adiabatically from k to 140 kpa and 400 k. what is the entropy change during this process?
Answers: 3

Engineering, 04.07.2019 18:10
Which of the following components of a pid controlled accumulates the error over time and responds to system error after the error has been accumulated? a)- proportional b)- derivative c)- integral d)- on/off.
Answers: 2

Engineering, 04.07.2019 18:20
Apiston-cylinder device contains 0.1 m3 of liquid water and 0.9 m3 of water vapor in equilibrium at 800 kpa. heat is transferred at constant pressure until the temperature of water reaches 350 °c. determine (a) the quality of water at the initial state (b) the work associated with this process, (c) the heat associated with this process.
Answers: 2
You know the right answer?
Using Raoult’s law, estimate the boiling temperature and mole fractions in the vapor phase that is i...
Questions