
Engineering, 24.07.2020 01:01 lanapope
Steam undergoes an isentropic compression in an insulatedpiston–cylinder assembly from an initial state where T1= 160°C, p1= 1 bar to a final state where the pressure p2= 30bar. Determinethe final temperature, in °C, and the work, in kJ per kg of steam

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 14:10
If the thermal strain developed in polyimide film during deposition is given as 0.0044. assume room temperature is kept at 17.3 c, and thermal coefficient of expansion for the film and the substrate are 54 x 10^-6c^-1 and 3.3 x 10^-6c^-1respectively. calculate the deposition temperature.
Answers: 3

Engineering, 03.07.2019 15:10
Heat is added to a piston-cylinder device filled with 2 kg of air to raise its temperature 400 c from an initial temperature of t1 27 cand pressure of pi 1 mpa. the process is isobaric process. find a)-the final pressure p2 b)-the heat transfer to the air.
Answers: 1

Engineering, 04.07.2019 18:10
Shafts are machine elements that are used to a) carry axial loads b) direct shear loads c) transmit power d) rotate at constant speed e) none of the above circular and square shafts subjected to the same torque under the same circum behave a) the same way b) almost the same way
Answers: 2

Engineering, 04.07.2019 18:10
An ideal otto cycle with air as the working fluid has a compression ratio of 8. the minimum and maximum temperatures in the cycle are 300 k and 1340 k. use constant specific heats at room temperature to determine (a) the amount of heat transferred to the air during the heat- addition kj/kg, (b) the thermal efficiency, and (c) the thermal efficiency of a carnot cycle ope limits. process, in rating between the same temperature
Answers: 2
You know the right answer?
Steam undergoes an isentropic compression in an insulatedpiston–cylinder assembly from an initial st...
Questions


Social Studies, 09.07.2019 06:30

English, 09.07.2019 06:30

Business, 09.07.2019 06:30

History, 09.07.2019 06:30

Mathematics, 09.07.2019 06:30

Business, 09.07.2019 06:30

Biology, 09.07.2019 06:30

Mathematics, 09.07.2019 06:30

Biology, 09.07.2019 06:30

Mathematics, 09.07.2019 06:30

Chemistry, 09.07.2019 06:30

Biology, 09.07.2019 06:30

Chemistry, 09.07.2019 06:30



Mathematics, 09.07.2019 06:30

Social Studies, 09.07.2019 06:30

Biology, 09.07.2019 06:30

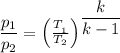
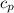 ×(T₂ - T₁)
×(T₂ - T₁) 

