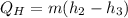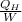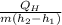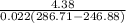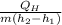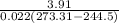Engineering, 05.07.2020 14:01 jazmyn1314
Refrigerant-134a enters the condenser of a residential heat pump at 800 kPa and 50°C at a rate of 0.02 kg/s and leaves at 750 kPa subcooled by 3°C. The refrigerant enters the compressor at 200 kPa superheated by 4°C. Determine the isentropic efficiency of the compressor, the rate of heat supplied to the room, COP of the Heat Pump and the rate of heat supplied to this room if the heat pump operated on an ideal vapor compression cycle between pressure limits of 200 and 800 kpa

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 12:10
On a average work day more than work place firs are reorted
Answers: 1

Engineering, 04.07.2019 19:10
What is the main objective of using reheat rankine cycle?
Answers: 3

Engineering, 04.07.2019 19:20
Acompressor compresses a gas, a pump compresses a liquid. for a given pressure ratio, why does it take more work to compress a gas in a compressor than a liquid in a pump? a)- for a given pressure ratio the average specific volume for a gas is much higher than the average specific volume for a liquid. b)- there is no difference. the only difference is the amount of heat generated (not work) c)- for a given pressure ratio the average volurge for a gas is much higher than the average volume for a liquid. d)-there is no difference
Answers: 3

Engineering, 06.07.2019 02:30
Air (c-1.006 kj/kg.k, r-0.287 kj/kg.k) enters a nozzle steadily at 280 kpa and 77°c with a velocity of 50 m/s and exits at 85 kpa and 320 m/s. the heat losses from the nozzle to the surrounding medium at 20°c are estimated to be 3.2 kj/kg. determine (a) the exit temperature and (b) the total entropy change for this process. solve this problem using constant specific heats.
Answers: 1
You know the right answer?
Refrigerant-134a enters the condenser of a residential heat pump at 800 kPa and 50°C at a rate of 0...
Questions



SAT, 29.10.2021 03:40

Mathematics, 29.10.2021 03:50


Mathematics, 29.10.2021 03:50


Social Studies, 29.10.2021 03:50







Computers and Technology, 29.10.2021 04:00

English, 29.10.2021 04:00


History, 29.10.2021 04:00


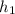 = 247.88 kJ/kg,
= 247.88 kJ/kg, 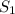 = 0.9579 kJ/kg K
= 0.9579 kJ/kg K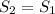 and given pressure at 2 with data from A-13 using interpolation is:
and given pressure at 2 with data from A-13 using interpolation is: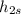 = 279.45 kJ/kg
= 279.45 kJ/kg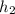 = 286.71 kJ/kg
= 286.71 kJ/kg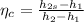
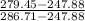
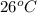 for given pressure as per saturated liquid approximation and data from A-11.
for given pressure as per saturated liquid approximation and data from A-11.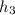 = 87.83 kJ/Kg
= 87.83 kJ/Kg