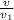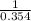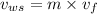Engineering, 04.07.2020 02:01 cheergirl2133
Steam is contained in a closed rigid container which has a volume of 2 initially the the pressure and the temperature is the remeraturedrops as a result of heat transfer to the surroundings. Determine
a) the temperature at which condensation first occurs, in °C,
b) the fraction of the total mass that has condensed when the pressure reaches 0.5 bar.
c) What is the volume, in m3, occupied by saturated liquid at the final state?

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 18:20
Wiy doeres rere okhn a pump whon working betwon the same pressure range?
Answers: 2

Engineering, 04.07.2019 19:10
The sum of the normal stresses does not change as the stress state rotates through an angle. a)-trune b)- false
Answers: 2

Engineering, 04.07.2019 19:10
The air in an automobile tire with a volume of 0.015 m3 is at 32°c and 140 kpa gage. determine the amount of air that must be added to raise the pressure to the recommended value of 206 kpa gage. assume the atmospheric pressure to be 128 kpa and the temperature and the volume to remain constant.[r-0.287 kj/kgk]
Answers: 3

Engineering, 04.07.2019 19:10
An engine, weighing 3000 n, is supported on a pedestal mount. it has been observed that the engine induces vibration into the surrounding area through its pedestal at the maximum operating speed. determine the stiffness of the dynamic vibration absorber spring in (n/m) that will reduce the vibration when mounted on the pedestal. the magnitude of the exciting force is 250 n, and the amplitude of motion of the auxiliary mass is to be limited to 2 mm note: in this question type-in right numbers, no decimals, no fractions, no unit. approximate to right number if needed
Answers: 3
You know the right answer?
Steam is contained in a closed rigid container which has a volume of 2 initially the the pressure an...
Questions




History, 13.05.2021 07:00



English, 13.05.2021 07:00




Mathematics, 13.05.2021 07:00


Physics, 13.05.2021 07:00

Mathematics, 13.05.2021 07:00

English, 13.05.2021 07:00



Mathematics, 13.05.2021 07:00

Mathematics, 13.05.2021 07:00

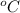 ,
, 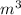 , occupied by saturated liquid at the final state?
, occupied by saturated liquid at the final state?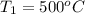 ,
, 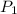 = 10 bar
= 10 bar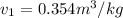
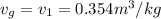
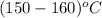
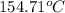
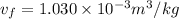
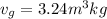
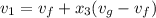
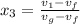
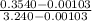
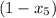 = 1 - 0.109
= 1 - 0.109