
Engineering, 18.06.2020 16:57 Csoard2004
The volume of 1.5 kg of helium in a frictionless piston-cylinder device is initially 6 m3. Now, helium is compressed to 2 m3 while its pressure is maintained constant at 200 kPa. Determine the initial and final temperatures of helium, as well as the work required to compress it, in kJ.

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 14:10
Explain the difference laminar and turbulent flow. explain it with the shear stress and the velocity profiles.
Answers: 1

Engineering, 03.07.2019 14:10
When at a point two solid phase changes to one solid phase on cooling then it is known as a) eutectoid point b) eutectic point c) peritectic point d) peritectoid point
Answers: 3

Engineering, 03.07.2019 14:10
Amass of 1.5 kg of air at 120 kpa and 24°c is contained in a gas-tight, frictionless piston-cylinder device. the air is now compressed to a final pressure of 720 kpa. during the process, heat is transferred from the air such that the temperature inside the cylinder remains constant. calculate the boundary work input during this process.
Answers: 2

Engineering, 03.07.2019 23:20
Two technicians are discussing the intake air temperature (iat) sensor. technician a says that the computer uses the iat sensor as a backup to the engine coolant temperature (ect) sensor. technician b says that the powertrain control module (pcm) will subtract the calculated amount of fuel if the air measures hot. who is correct
Answers: 3
You know the right answer?
The volume of 1.5 kg of helium in a frictionless piston-cylinder device is initially 6 m3. Now, heli...
Questions



Mathematics, 22.09.2020 19:01





Computers and Technology, 22.09.2020 19:01



Mathematics, 22.09.2020 19:01



Biology, 22.09.2020 19:01

English, 22.09.2020 19:01



Mathematics, 22.09.2020 19:01

Mathematics, 22.09.2020 19:01

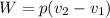
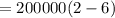
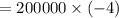
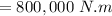
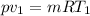

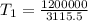
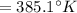 (Initial temperature of helium)
(Initial temperature of helium)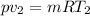
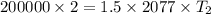
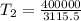
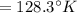 (Final temperature of helium)
(Final temperature of helium)

