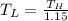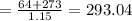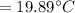Engineering, 29.05.2020 20:00 sassyunicorngir
Consider a Carnot heat pump cycle executed in a steady-flow system in the saturated mixture region using R-134a flowing at a rate of 0.264 kg/s. The maximum absolute temperature in the cycle is 1.15 times the minimum absolute temperature, and the net power input to the cycle is 5 kW. If the refrigerant changes from saturated vapor to saturated liquid during the heat rejection process, determine the ratio of the maximum to minimum pressures in the cycle.

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 14:10
The y form of iron is known as: a) ferrite b) cementite c) perlite d) austenite
Answers: 3

Engineering, 04.07.2019 18:10
Air is to be cooled in the evaporator section of a refrigerator by passing it over a bank of 0.8-cm-outer-diameter and 0.4-m-long tubes inside which the refrigerant is evaporating at -20°c. air approaches the tube bank in the normal direction at 0°c and 1 atm with a mean velocity of 4 m/s. the tubes are arranged in-line with longitudinal and transverse pitches of sl- st 1.5 cm. there are 30 rows in the flow direction with 15 tubes in each row. determine (a) the refrigeration capacity of this system and (b) pressure drop across the tube bank. evaluate the air properties at an assumed mean temperature of -5°c and 1 atm. is this a good assumption?
Answers: 1

Engineering, 04.07.2019 18:10
Coiled springs ought to be very strong and stiff. si3n4 is a strong, stiff material. would you select this material for a spring? explain.
Answers: 2

Engineering, 04.07.2019 18:10
During a steady flow process, the change of energy with respect to time is zero. a)- true b)- false
Answers: 2
You know the right answer?
Consider a Carnot heat pump cycle executed in a steady-flow system in the saturated mixture region u...
Questions

Mathematics, 05.07.2020 17:01

Mathematics, 05.07.2020 17:01




Biology, 05.07.2020 17:01

Mathematics, 05.07.2020 17:01

Mathematics, 05.07.2020 17:01


Mathematics, 05.07.2020 17:01

Mathematics, 05.07.2020 17:01

Social Studies, 05.07.2020 17:01

Mathematics, 05.07.2020 17:01







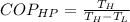
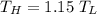
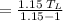
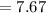
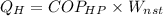
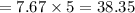
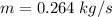
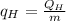
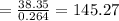
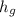 value is 145.27 and therefore the hot reservoir temperature is 64° C.
value is 145.27 and therefore the hot reservoir temperature is 64° C.