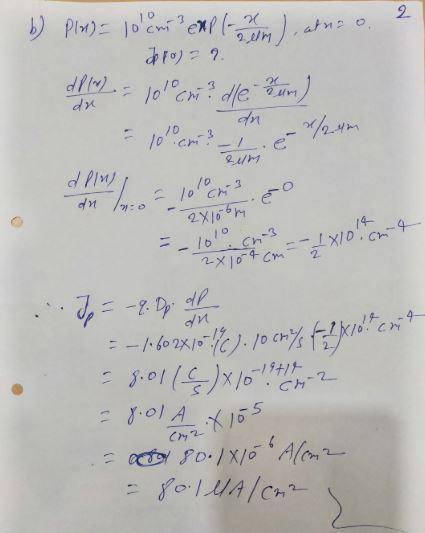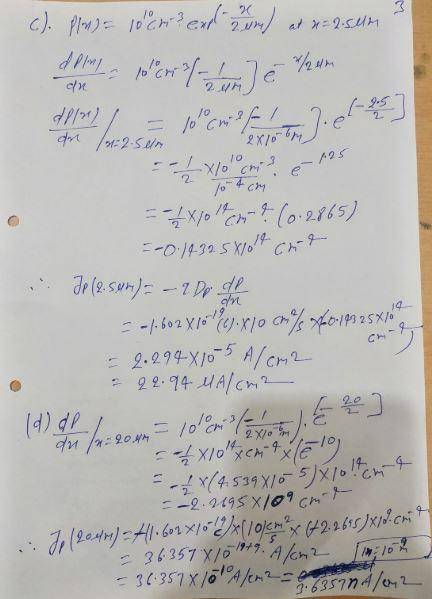Engineering, 20.04.2020 22:41 notcollin2416
Calculate the diffusion current density for the following carrier distributions. For electrons, use Dn = 35 cm2/s and for holes, use Dp = 10 cm2/s. a. ; Jn = b. , at x = 0: Jp (0) = c. , at x = 2.5 µm: Jp (2.5 µm) = d. , at x = 20 µm: Jp (20 µm) = e. , at x = 5 µm: Jn (5 µm) = n (x) = (1010 cm−3 ) 5 μm − x 5 μm

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 18:10
Apump is used to circulate hot water in a home heating system. water enters the well-insulated pump operating at steady state at a rate of 0.42 gal/min. the inlet pressure and temperature are 14.7 lbf/in.2, and 180°f, respectively; at the exit the pressure is 60 lbf/in.2 the pump requires 1/15 hp of power input. water can be modeled as an incompressible substance with constant density of 60.58 lb/ft3 and constant specific heat of 1 btu/lb or. neglecting kinetic and potential energy effects, determine the temperature change, in °r, as the water flows through the pump.
Answers: 1

Engineering, 04.07.2019 18:10
Calculate the bore of a cylinder that has a stroke of 18 inches and an extension time of 6 seconds at a flow rate of 4 gal/min.
Answers: 3

Engineering, 04.07.2019 18:20
Select any two (2) areas of applications of chain-drive. (clo4) a)-permanent lubrication necessary b)-hydraulic forklift truck operation c)-rigging and heavy moving materials d)-relatively high maintenance costs e)-costlier than belt drives
Answers: 2

Engineering, 04.07.2019 18:20
Ahe-xe mixture containing a 0.75 mole fraction of helium is used for cooling electronics in an avionics application. at a temperature of 300 k and atmospheric pressure, calculate the mass fraction of helium and the mass density, molar concentration and molecular weight of the mixture. if the cooling capacity is 10 l, what is the mass of the coolant?
Answers: 3
You know the right answer?
Calculate the diffusion current density for the following carrier distributions. For electrons, use...
Questions




Mathematics, 01.08.2019 11:00




Spanish, 01.08.2019 11:00

Mathematics, 01.08.2019 11:00

Social Studies, 01.08.2019 11:00




Mathematics, 01.08.2019 11:00

Mathematics, 01.08.2019 11:00

Mathematics, 01.08.2019 11:00

Biology, 01.08.2019 11:00

Mathematics, 01.08.2019 11:00

Biology, 01.08.2019 11:00

Mathematics, 01.08.2019 11:00