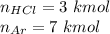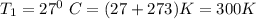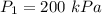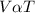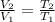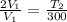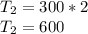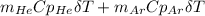Engineering, 11.04.2020 03:45 raulriquelmef6p0947w
A piston-cylinder device contains an ideal gas mixture of 3 kmol of He gas and 7 kmol of Ar gas (both gases are monatomic) at 27°C and 200 kPa. Now the gas expands at constant pressure until its volume doubles. The amount of heat transfer to the gas mixture is, in MJ (round to nearest integer; for example if the answer is 53.7MJ, write 54; if the answer is 52.1MJ write 52; do not include the units in your answer)

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 18:10
The drive force for diffusion is 7 fick's first law can be used to solve the non-steady state diffusion. a)-true b)-false
Answers: 1

Engineering, 04.07.2019 18:10
The thermal expansion or contraction of a given metal is a function of the f a)-density b)-initial temperature c)- temperature difference d)- linear coefficient of thermal expansion e)- final temperature f)- original length
Answers: 2

Engineering, 04.07.2019 18:10
Which of the following controllers anticipates the future from the slope of errors over time? a)-proportional b)-on/off c)-integral d)-derivative.
Answers: 2

Engineering, 04.07.2019 18:20
Ahe-xe mixture containing a 0.75 mole fraction of helium is used for cooling electronics in an avionics application. at a temperature of 300 k and atmospheric pressure, calculate the mass fraction of helium and the mass density, molar concentration and molecular weight of the mixture. if the cooling capacity is 10 l, what is the mass of the coolant?
Answers: 3
You know the right answer?
A piston-cylinder device contains an ideal gas mixture of 3 kmol of He gas and 7 kmol of Ar gas (bot...
Questions

Mathematics, 10.02.2021 05:20


Mathematics, 10.02.2021 05:20

Mathematics, 10.02.2021 05:20



Mathematics, 10.02.2021 05:20

History, 10.02.2021 05:20

English, 10.02.2021 05:20


Chemistry, 10.02.2021 05:20

History, 10.02.2021 05:20

Mathematics, 10.02.2021 05:20



Social Studies, 10.02.2021 05:20

Geography, 10.02.2021 05:20


Mathematics, 10.02.2021 05:20

Mathematics, 10.02.2021 05:20