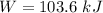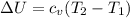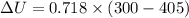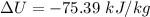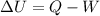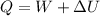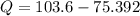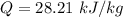Engineering, 06.04.2020 23:33 meadowsoares7
Nitrogen (N2) contained in a piston–cylinder arrangement, initially at 10 bar and 405 K, undergoes an expansion to a final temperature of 300 K, during which the pressure–volume relationship is pV1.3 = constant. Assuming the ideal gas model for the N2, determine the heat transfer in kJ/kg.

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 18:10
Steel is coated with a thin layer of ceramic to protect against corrosion. what do you expect to happen to the coating when the temperature of the steel is increased significantly? explain.
Answers: 1

Engineering, 04.07.2019 18:10
At 12 noon, the count in a bacteria culture was 400; at 4: 00 pm the count was 1200 let p(t) denote the bacteria cou population growth law. find: (a) an expression for the bacteria count at any time t (b) the bacteria count at 10 am. (c) the time required for the bacteria count to reach 1800.
Answers: 1

Engineering, 04.07.2019 18:20
Athin walled concentric tube exchanger is used to cool engine oil from 160°c to 60°c with water that is available at 25°c acting as a coolant. the oil and water flow rates are each at 2 kg/s, and the diameter of the inner tube is 0.5 m and the corresponding value of the overall heat transfer coefficient is 250 w/m2. oc. how long must the heat exchanger be to accomplish the desired cooling? cpwater=4.187 kj/kg-candcpengine el=2.035 kj/kg·°c, oil . 120]
Answers: 1

Engineering, 04.07.2019 19:20
Heat transfer by is the fastest mode of heat transfer that requires no intervening medium. a)-conduction b)-convection c)-radiation d)-conduction and convection
Answers: 1
You know the right answer?
Nitrogen (N2) contained in a piston–cylinder arrangement, initially at 10 bar and 405 K, undergoes a...
Questions





Mathematics, 08.04.2020 02:11



Biology, 08.04.2020 02:11









Law, 08.04.2020 02:11



Mathematics, 08.04.2020 02:12

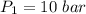
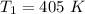
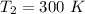
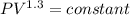
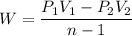
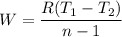
![W=\dfrac{0.296(405-300)}{1.3-1}\quad [R_{N_2}=\frac{8.314}{28}]](/tpl/images/0585/1869/e6619.png)