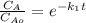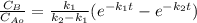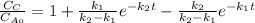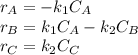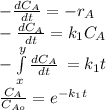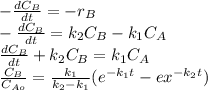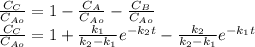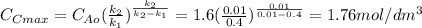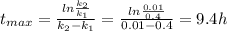The elementary liquid-phase series reaction
A k1> B k2> C
is carried out in a 500-...

Engineering, 30.03.2020 23:05 avinashpolwah
The elementary liquid-phase series reaction
A k1> B k2> C
is carried out in a 500-dm^3 batch reactor. The initial concentration of A is 1.6 mol/dm^3. The desired product is B, and separation of the undesired product C is very difficult and costly. Because the reaction is carried out at a relatively high temperature, the reaction is easily quenched.
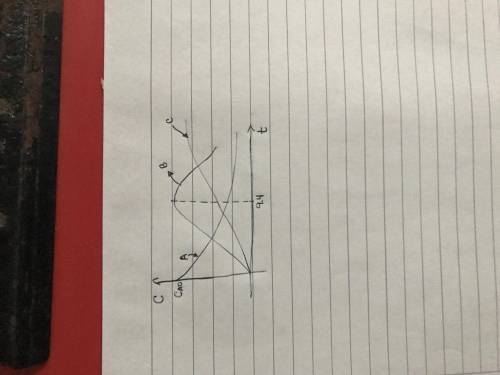
(a) Plot and analyze the concentrations of A, B, and C as a function of time. Assume that each reaction is irreversible, with k1 = 0.4 h^-1 and k2 = 0.01 h^-1.

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 18:10
What difference(s) did you notice using a pneumatic circuit over hydraulic circuit.explain why the pneumatic piston stumbles when it hits an obstacle.
Answers: 2

Engineering, 04.07.2019 18:10
Acompressor receives the shaft work to decrease the pressure of the fluid. a)- true b)- false
Answers: 3

Engineering, 04.07.2019 18:10
The higher the astm grain-size number, the coarser the grain is. a)-true b)-false
Answers: 3

Engineering, 04.07.2019 18:20
Athin walled concentric tube exchanger is used to cool engine oil from 160°c to 60°c with water that is available at 25°c acting as a coolant. the oil and water flow rates are each at 2 kg/s, and the diameter of the inner tube is 0.5 m and the corresponding value of the overall heat transfer coefficient is 250 w/m2. oc. how long must the heat exchanger be to accomplish the desired cooling? cpwater=4.187 kj/kg-candcpengine el=2.035 kj/kg·°c, oil . 120]
Answers: 1
You know the right answer?
Questions


Mathematics, 02.12.2020 03:10

Biology, 02.12.2020 03:10

Mathematics, 02.12.2020 03:10



Mathematics, 02.12.2020 03:10


Mathematics, 02.12.2020 03:10


History, 02.12.2020 03:10

Social Studies, 02.12.2020 03:10

Mathematics, 02.12.2020 03:10



Computers and Technology, 02.12.2020 03:10


Advanced Placement (AP), 02.12.2020 03:10

Mathematics, 02.12.2020 03:10

Mathematics, 02.12.2020 03:10