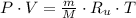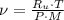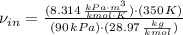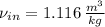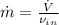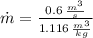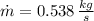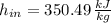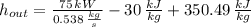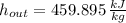Engineering, 30.03.2020 19:21 paigesyring
Air enters a compressor operating at steady state with pressure of 90 kPa, at a temperature of 350 K, and a volumetric flow rate of 0.6 m3/s. The air exits the compressor at a pressure of 700 kPa. Heat transfer from the compressor to its surrounding occurs at a rate of 30 kJ/kg of air flowing. The compressor power input is 75 kW. Neglecting kinetic and potential energy effects and assuming air to be an ideal gas, find the exit temperature of air.

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 18:10
Manometers are good examples of measuring instruments, nowadays they are not as common as before. a)-capacitive probe gauges b)-gravitational gauges deformation ) gauges d)-digital gauges
Answers: 1

Engineering, 04.07.2019 19:10
10 kg of co2 is initially contained at 400 kpa and 300 k. the gas constant for carbon dioxide is 189 j/lkg k) and has a specific heat ratio, k, of 1.289. isentropic expansion then occurs until the pressure is 200 kpa. a) determine the initial volume of co2 in m. b) determine the final temperature in k. c) determine the work done by the system during the expansion kl.
Answers: 2

Engineering, 04.07.2019 19:10
Apressure vessel with an r/t 20 cannot be treated as thin walled vessel. a)-trune b)- false
Answers: 3

Engineering, 04.07.2019 19:20
A5 kg block of fe is dropped into a very large vat of water. the fe and water initial temperatures are 95 and 25 c, respectively. the fe final temperature is 25 c and the water can be treated as a thermal reservoir,. treated as a thermal reservoir take the water to be the system and determine the entropy generation. report vour answer in kj/k.
Answers: 1
You know the right answer?
Air enters a compressor operating at steady state with pressure of 90 kPa, at a temperature of 350 K...
Questions




Computers and Technology, 30.09.2019 15:00

Mathematics, 30.09.2019 15:00




English, 30.09.2019 15:00


Physics, 30.09.2019 15:00






History, 30.09.2019 15:00

Mathematics, 30.09.2019 15:00


Mathematics, 30.09.2019 15:00

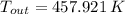
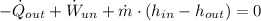
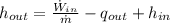
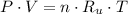
 - Absolute pressure, in kilopascals.
- Absolute pressure, in kilopascals. - Volume, in cubic meters.
- Volume, in cubic meters.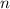 - Quantity of moles, in kilomole.
- Quantity of moles, in kilomole.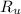 - Ideal gas universal constant, in
- Ideal gas universal constant, in 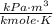 .
.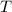 - Absolute temperature, in kelvin.
- Absolute temperature, in kelvin.