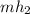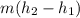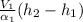Engineering, 25.03.2020 06:28 babas97
Refrigerant-134a enters an adiabatic compressor at 100 kPa and –24°C with a flow rate of 1.25 m3/min and leaves at 800 kPa and 60°C. Determine the mass flow rate of R-134a and the power input to the compressor. Use data from the steam tables. Heat transfer with the surroundings is negligible. Neglect kinetic and potential energy changes.

Answers: 3


Another question on Engineering

Engineering, 03.07.2019 15:10
Heat is added to a piston-cylinder device filled with 2 kg of air to raise its temperature 400 c from an initial temperature of t1 27 cand pressure of pi 1 mpa. the process is isobaric process. find a)-the final pressure p2 b)-the heat transfer to the air.
Answers: 1

Engineering, 03.07.2019 15:10
Two flowing streams of argon gas are adiabatically mixed to form a single flow/stream. one stream is 1.5 kg/s at 400 kpa and 200 c while the second stream is 2kg/s at 500 kpa and 100 ? . it is stated that the exit state of the mixed single flow of argon gas is 150 c and 300 kpa. assuming there is no work output or input during the mixing process, does this process violate either the first or the second law or both? explain and state all your assumptions.
Answers: 1

Engineering, 04.07.2019 08:10
Which of the following is an easy way to remember the modified “x” tire rotation? a. nondrive wheels straight, cross the drive wheels b. drive wheels straight, cross the nondrive wheels c. drive wheels crossed, nondrive wheels straight d. drive wheels crossed, nondrive wheels crossed
Answers: 1

Engineering, 04.07.2019 18:10
The drive force for diffusion is 7 fick's first law can be used to solve the non-steady state diffusion. a)-true b)-false
Answers: 1
You know the right answer?
Refrigerant-134a enters an adiabatic compressor at 100 kPa and –24°C with a flow rate of 1.25 m3/min...
Questions


History, 02.10.2019 17:30

History, 02.10.2019 17:30

Mathematics, 02.10.2019 17:30



Mathematics, 02.10.2019 17:30

Mathematics, 02.10.2019 17:30


Mathematics, 02.10.2019 17:30


History, 02.10.2019 17:30


Mathematics, 02.10.2019 17:30



English, 02.10.2019 17:30

Computers and Technology, 02.10.2019 17:30

Business, 02.10.2019 17:30

Mathematics, 02.10.2019 17:30

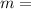
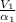
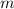
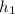 + W =
+ W =