
Engineering, 13.03.2020 03:33 jamesgotqui6
One kilogram of air, initially at 5 bar, 350 K, and 3 kg of carbon dioxide (CO2), initially at 2 bar, 450 K, are confined to opposite sides of a rigid, well-insulated container. The partition is free to move and allows conduction from one gas to the other without energy storage in the partition itself. The air and carbon dioxide each behave as ideal gases.
Determine the final equilibrium temperature, in K, and the final pressure, in bar, assuming constant specific heats.

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 14:10
If the thermal strain developed in polyimide film during deposition is given as 0.0044. assume room temperature is kept at 17.3 c, and thermal coefficient of expansion for the film and the substrate are 54 x 10^-6c^-1 and 3.3 x 10^-6c^-1respectively. calculate the deposition temperature.
Answers: 3

Engineering, 03.07.2019 19:30
When using the ohmmeter function of a digital multimeter, the leads are placed in what position relative to the component being tested? a. parallel b. control c. series d. line
Answers: 3

Engineering, 04.07.2019 03:10
What precautions should you take to prevent injuries when dealing with heavy loads?
Answers: 1

Engineering, 04.07.2019 18:10
You are making beer. the first step is filling the glass carboy with the liquid wort. the internal diameter of the carboy is 15 in., and you wish to fill it up to a depth of 2 ft. if your wort is drawn from the kettle using a siphon process that flows at 3 gpm, how long will it take to fill?
Answers: 1
You know the right answer?
One kilogram of air, initially at 5 bar, 350 K, and 3 kg of carbon dioxide (CO2), initially at 2 bar...
Questions



Health, 04.07.2019 07:00



Mathematics, 04.07.2019 07:00

Mathematics, 04.07.2019 07:00

Mathematics, 04.07.2019 07:00

Mathematics, 04.07.2019 07:00




Health, 04.07.2019 07:00

Mathematics, 04.07.2019 07:00

Mathematics, 04.07.2019 07:00

Mathematics, 04.07.2019 07:00


Mathematics, 04.07.2019 07:00

Mathematics, 04.07.2019 07:00

Mathematics, 04.07.2019 07:00

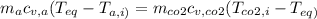
 =3x0.780x
=3x0.780x ⇒
⇒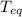 = 426.4 °K
= 426.4 °K 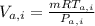 =
= 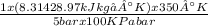 = 0.201
= 0.201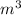
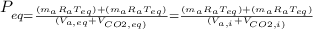
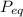 = 1x(8.314 28.97)x426.4+3x(8.314 44)x426.4
= 1x(8.314 28.97)x426.4+3x(8.314 44)x426.4

