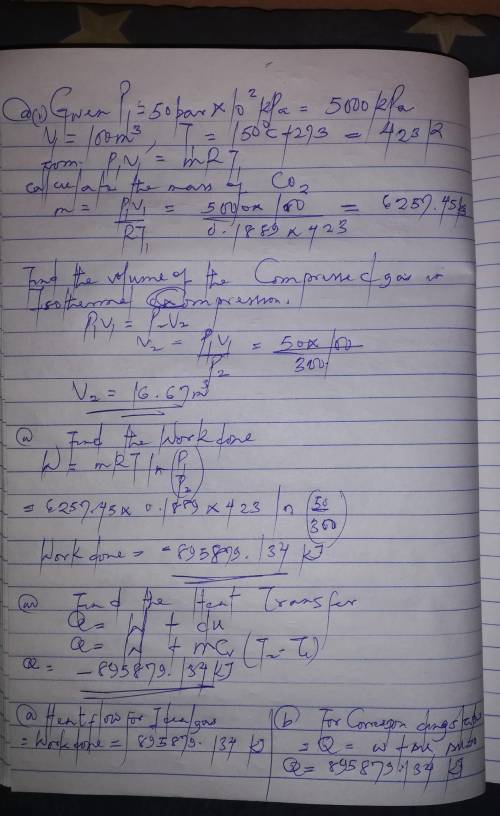Engineering, 07.03.2020 05:56 Schoolwork100
100 cubic meters of carbon dioxide initially at 150 C and 50bar is to be isothermally compressed in a frictionless piston and cylinder device to a final pressure of 300 bar. Calculate:
i. The volume of the compressed gas
ii. The work done to compress the gas
iii. The heat flow on compression assuming carbon dioxide:
a/ Is an ideal gas
b/ Obeys the principle of corresponding states
c/ Obeys the Peng-RObinson equation of state

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 16:10
An electrical motor raises a 50kg load at a construct velencity .calculate the power of the motor, if it takes 40sec to raise the load through a height of 24m(take g =9.8n/g)
Answers: 2

Engineering, 04.07.2019 18:10
Apump is used to circulate hot water in a home heating system. water enters the well-insulated pump operating at steady state at a rate of 0.42 gal/min. the inlet pressure and temperature are 14.7 lbf/in.2, and 180°f, respectively; at the exit the pressure is 60 lbf/in.2 the pump requires 1/15 hp of power input. water can be modeled as an incompressible substance with constant density of 60.58 lb/ft3 and constant specific heat of 1 btu/lb or. neglecting kinetic and potential energy effects, determine the temperature change, in °r, as the water flows through the pump.
Answers: 1

Engineering, 04.07.2019 18:10
Afluid flows with a velocity field given by v=(x/t)i.. determine the local and convective accelerations when x=3 and t=1.
Answers: 2

Engineering, 04.07.2019 18:10
A-mn has a cubic structure with a0 0.8931 nm and a density of 7.47 g/cm3. b-mn has a different cubic structure, with a0 0.6326 nm and a density of 7.26 g/cm3. the atomic weight of manganese is 54.938 g/mol and the atomic radius is 0.112 nm. determine the percent volume change that would occur if a-mn transforms to b-mn.
Answers: 2
You know the right answer?
100 cubic meters of carbon dioxide initially at 150 C and 50bar is to be isothermally compressed in...
Questions

Mathematics, 08.04.2020 04:23






History, 08.04.2020 04:23












Physics, 08.04.2020 04:23

Health, 08.04.2020 04:23