
Engineering, 05.03.2020 12:32 ErickP1686
Two soils are fully saturated with liquid (no gas present) and the soils have the same void ratio. One soil is saturated with water and the other is saturated with alcohol. The unit weight of water is approximately 1g/cm3 while that of alcohol is about 0.8 g/cm3. Which soil sample has the larger water content? Why?

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 18:10
An air conditioning system consist of a 5 cm diameter pipe, operating at a pressure of 200 kpa. the air initially enters the pipe at 15°c with a velocity of 20 m/s and relative humidity of 80%. if the heat supply throughout the process is 960 w, determine the relative humidity and the temperature at the outlet
Answers: 3

Engineering, 04.07.2019 18:10
Different types of steels contain different elements that alter the characteristics of the steel. for each of the following elements, explain what the element does when alloyed with steel.
Answers: 2

Engineering, 04.07.2019 18:20
Find the kinematic pressure of 160kpa. for air, r-287 j/ kg k. and hair al viscosity of air at a temperature of 50°c and an absolute (10 points) (b) find the dynamic viscosity of air at 110 °c. sutherland constant for air is 111k
Answers: 3

Engineering, 04.07.2019 18:20
An open feedwater heater operates at steady state with liquid entering at inlet 1 with t? = 40°c and pl = 1 .2 mpa. water vapor att2-200°c and p2 = 1.2 mpa enters at inlet 2. saturated liquid water exits with a pressure of pa 1.2 mpa. neglect heat transfer with the surroundings and all kinetic and potential energy effects, determine the mass flow rate of steam at inlet 2 if the mass flow rate of liquid water at inlet 1 is given as 2 kg/s.
Answers: 3
You know the right answer?
Two soils are fully saturated with liquid (no gas present) and the soils have the same void ratio. O...
Questions

History, 21.10.2020 04:01


French, 21.10.2020 04:01

Mathematics, 21.10.2020 04:01

Chemistry, 21.10.2020 04:01







Chemistry, 21.10.2020 04:01

History, 21.10.2020 04:01




Mathematics, 21.10.2020 04:01

French, 21.10.2020 04:01

Mathematics, 21.10.2020 04:01

Mathematics, 21.10.2020 04:01

 ....................1
....................1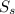 is 100% in both case
is 100% in both case 

