Engineering, 26.02.2020 05:58 erikagibson3414
A perfect gas expands in a frictionless nozzle from stagnation conditions p 0 = 4 MPa, T0 = 2500 K to ambient pressure 0.1 MPa. Given that the expansion is isentropic, determine the following conditions at the final pressure: (1) velocity, (2) Mach number, (3) temperature, and (4) area per unit mass flow. How does the final flow area compare with the throat area for a PROBLEMS 89 given mass flow? The specific heat ratio y is 1.4, and the molecular weight M is 30.

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 18:10
Manometers are good examples of measuring instruments, nowadays they are not as common as before. a)-capacitive probe gauges b)-gravitational gauges deformation ) gauges d)-digital gauges
Answers: 1

Engineering, 04.07.2019 18:20
Asolid cylinder is concentric with a straight pipe. the cylinder is 0.5 m long and has an outside diameter of 8 cm. the pipe has an inside diameter of 8.5 cm. the annulus between the cylinder ad the pipe contains stationary oil. the oil has a specific gravity of 0.92 and a kinematic viscosity of 5.57 x 10-4 m2/s. most nearly, what is the force needed to move the cylinder along the pipe at a constant velocity of 1 m/s?
Answers: 3

Engineering, 04.07.2019 18:20
Determine the damped natural frequencies and the steady state response of a decoupled damped forced two degrees of freedom system. 10ä1 + 2q1 20q1 10 cos t; 10q2 +4q2 + 40q2 10 cos t
Answers: 3

Engineering, 04.07.2019 18:20
An engine runs on the ideal diesel cycle. the cycle has a compression ratio of 20 and a cutoff ratio of 2. the highest temperature in the cycle is 1200 k. if the heat into the system is 300 kj/kg of working fluid and using variable specific heats determine the work produced per mass of working fluid
Answers: 3
You know the right answer?
A perfect gas expands in a frictionless nozzle from stagnation conditions p 0 = 4 MPa, T0 = 2500 K t...
Questions

Biology, 25.01.2022 02:20

Mathematics, 25.01.2022 02:20



Mathematics, 25.01.2022 02:20




Mathematics, 25.01.2022 02:20


Mathematics, 25.01.2022 02:20






Mathematics, 25.01.2022 02:20

Mathematics, 25.01.2022 02:20


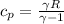 = 1.005 kJ/kg
= 1.005 kJ/kg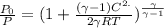
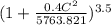 or c = 164.108 m/s
or c = 164.108 m/s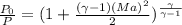 That is
That is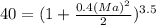 or
or