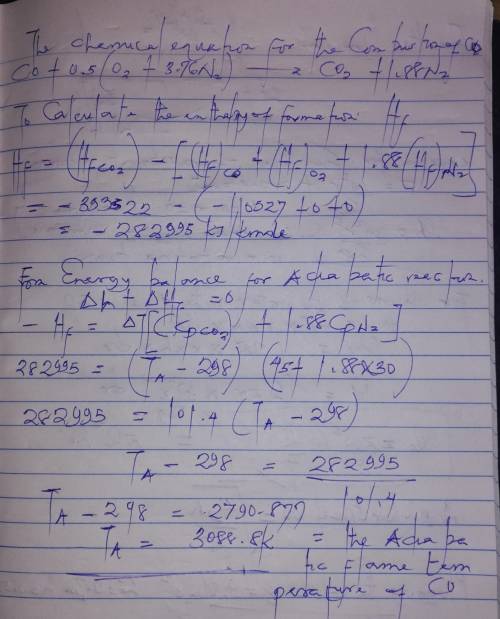Engineering, 21.02.2020 20:53 shaydog6353
Determine the adiabatic flame temperature of carbon monoxide (CO) burning in air at an equivalence ratio of unity. The reactants are at standard temperature and pressure. Assume constant specific heats.

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 18:10
The higher the astm grain size number, the finer the gran is. a)-true b)-false
Answers: 2

Engineering, 04.07.2019 18:20
Ahe-xe mixture containing a 0.75 mole fraction of helium is used for cooling electronics in an avionics application. at a temperature of 300 k and atmospheric pressure, calculate the mass fraction of helium and the mass density, molar concentration and molecular weight of the mixture. if the cooling capacity is 10 l, what is the mass of the coolant?
Answers: 3

Engineering, 04.07.2019 18:20
Air flows over a heated plate àt a velocity of 50m/s. the local skin factor coefficient at a point on a plate is 0.004. estimate the local heat transfer coefficient at this point.the following property data for air are given: density = 0.88kg/m3 , viscosity 2.286 x 10 ^-5 kgm/s , k = 0.035w/mk ,cp = 1.001kj/kgk. use colburn reynolds analogy.
Answers: 1

Engineering, 06.07.2019 02:30
Plot schematically the tensile stress versus strain curves for a typical thermoplastic material at a temperature below its glass transition temperature (tg and at a temperature above its tg, respectively. do the same for a typical thermosetting material. list in a table any differences or similarities between the two materials at t> tg and t < tg, respectively, and relate them to the structures of the two types of polymers
Answers: 3
You know the right answer?
Determine the adiabatic flame temperature of carbon monoxide (CO) burning in air at an equivalence r...
Questions


History, 17.07.2019 17:00

Mathematics, 17.07.2019 17:00

Biology, 17.07.2019 17:00




History, 17.07.2019 17:00

Mathematics, 17.07.2019 17:00

Mathematics, 17.07.2019 17:00

English, 17.07.2019 17:00


History, 17.07.2019 17:00




Mathematics, 17.07.2019 17:00

Advanced Placement (AP), 17.07.2019 17:00


Physics, 17.07.2019 17:00