Engineering, 21.02.2020 18:42 saeedalr366
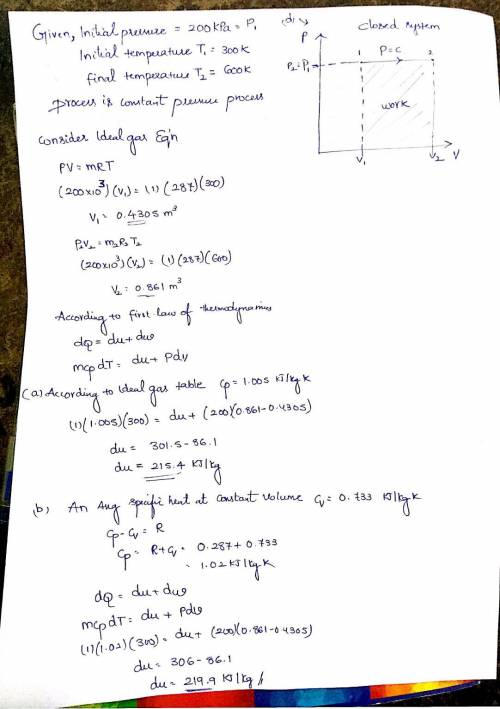
Air, in a piston cylinder assembly, is initially at 300 K and 200 kPa. It is then heated at constant pressure to 600 K. Determine the change in internal energy of air per unit mass, using:
a) The ideal gas table for air. b) Show the process on a (p-v) diagram.

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 18:10
The filament of an incandescent lamp has a temperature of 2000k. calculate the fraction of radiation emitted in the visible light band if the filament is approximated as blackbody
Answers: 2

Engineering, 04.07.2019 18:10
Carbon dioxide gas expands isotherm a turbine from 1 mpa, 500 k at 200 kpa. assuming the ideal gas model and neglecting the kinetic and potential energies, determine the change in entropy, heat transfer and work for each kilogram of co2.
Answers: 2

Engineering, 04.07.2019 18:10
Assuming compressible flow of air and that the measurements are done at flagstaff a pitot static tube that gives the difference of total and static pressure measures 0.35 m of mercury. what is the velocity of air? assume the temperature to be 300k. (submit your excel or matlab calculation sheet)
Answers: 1

Engineering, 04.07.2019 18:10
Which one from below is not one of the reasons of planning failures? (clo3) a)-planner is careless. b-planner spend less time in the field but more time on the desk c)-planner is not qualified d)-planner does not have sufficient time to properly plan
Answers: 3
You know the right answer?
Air, in a piston cylinder assembly, is initially at 300 K and 200 kPa. It is then heated at constant...
Questions

Mathematics, 21.05.2021 22:00


Mathematics, 21.05.2021 22:00

Mathematics, 21.05.2021 22:00

English, 21.05.2021 22:00

Mathematics, 21.05.2021 22:00



Mathematics, 21.05.2021 22:00

Biology, 21.05.2021 22:00




Mathematics, 21.05.2021 22:00


Mathematics, 21.05.2021 22:00


Geography, 21.05.2021 22:00