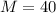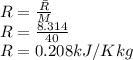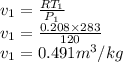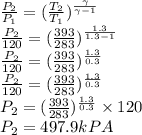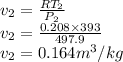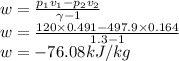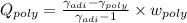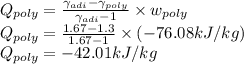Engineering, 21.02.2020 02:36 queenjade2614
Argon is compressed in a polytropic process with = 1:3 (i. e., PV = costant) from 120 kPa and 10 C until it reaches a temperature of 120 C in a piston{cylinder device. Model the system as an ideal gas and determine the speci c work (work per unit mass) done by the gas and the heat per unit mass transferred during this the process.

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 18:10
Different types of steels contain different elements that alter the characteristics of the steel. for each of the following elements, explain what the element does when alloyed with steel.
Answers: 2

Engineering, 04.07.2019 18:10
The filament of an incandescent lamp has a temperature of 2000k. calculate the fraction of radiation emitted in the visible light band if the filament is approximated as blackbody
Answers: 2

Engineering, 04.07.2019 18:20
For each of the following process: a) sketch the p-v diagram, b)sketch t-s diagram, c) sketch t-v diagram, d) sketch the boundary work on one of the diagrams (a, b or c) and e) sketch the reversible heat transfer on one of the diagrams (a, b or c): 1- isobaric process from compressed liquid to superheated vapor 2- isothermal process from compressed liquid to superheated vapor 3- isentropic process from compressed liquid to superheated vapor
Answers: 3

Engineering, 04.07.2019 18:20
Water vapor initially at 10 bar and 400 °c is contained within a piston-cylinder assembly. the water lost heat to the surrounding according to isochoric (iso-volumetric) process until its temperature is 150 °c. the water is then condensed isothermally to saturated liquid. for the water as a system, calculate the work in kj/kg
Answers: 2
You know the right answer?
Argon is compressed in a polytropic process with = 1:3 (i. e., PV = costant) from 120 kPa and 10 C u...
Questions


English, 07.04.2021 19:40

Mathematics, 07.04.2021 19:40

English, 07.04.2021 19:40



Mathematics, 07.04.2021 19:40

Mathematics, 07.04.2021 19:40

Mathematics, 07.04.2021 19:40

Chemistry, 07.04.2021 19:40


Mathematics, 07.04.2021 19:40



Mathematics, 07.04.2021 19:40


Mathematics, 07.04.2021 19:40