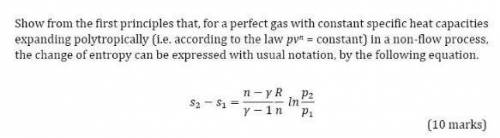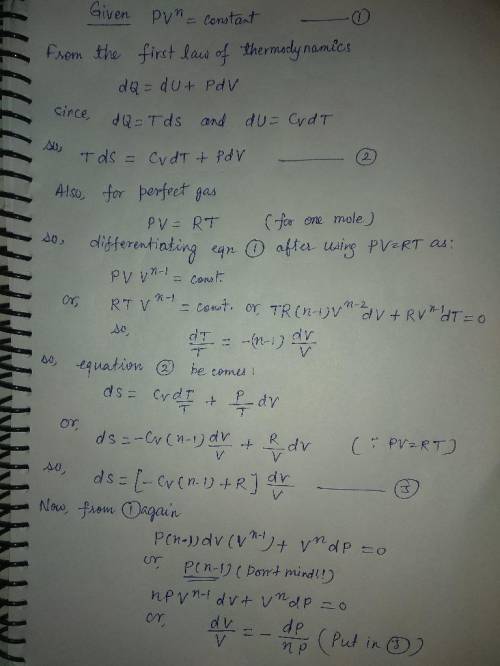Show from the first principles that, for a perfect gas with constant specific heat capacities
...

Engineering, 20.02.2020 11:14 cxndii13
Show from the first principles that, for a perfect gas with constant specific heat capacities
expanding polytropically (i. e. according to the law pvn = constant) in a non-flow process,
the change of entropy can be expressed with usual notation, by the following equation.
2 − 1 =( − / − 1)(/)(2/1)

Answers: 3


Another question on Engineering

Engineering, 03.07.2019 14:10
Explain the difference laminar and turbulent flow. explain it with the shear stress and the velocity profiles.
Answers: 1

Engineering, 04.07.2019 18:10
Steel is coated with a thin layer of ceramic to protect against corrosion. what do you expect to happen to the coating when the temperature of the steel is increased significantly? explain.
Answers: 1

Engineering, 04.07.2019 18:10
During a steady flow process, the change of energy with respect to time is zero. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:20
Inspection for bearing condition will include: (clo4) a)-color b)-smell c)-size d)-none of the above
Answers: 1
You know the right answer?
Questions

Computers and Technology, 26.08.2019 18:00


English, 26.08.2019 18:00


Mathematics, 26.08.2019 18:00

Mathematics, 26.08.2019 18:00

Mathematics, 26.08.2019 18:00



Spanish, 26.08.2019 18:00

Mathematics, 26.08.2019 18:00


Computers and Technology, 26.08.2019 18:00

Mathematics, 26.08.2019 18:00

Mathematics, 26.08.2019 18:00

English, 26.08.2019 18:00

Mathematics, 26.08.2019 18:00


Mathematics, 26.08.2019 18:00

Biology, 26.08.2019 18:00