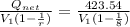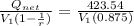Engineering, 06.02.2020 01:49 LilDicky
An ideal otto cycle has a compression ratio of 8. at the begining of the compression process, air is at 95 kpa and 27 c, and 750kj/kg of heat is transferred to air during the constant volume heat addition process taking into account the variation of specific heats with temperature, determine
(a) the pressure and temperature at the end of the heataddition process,
(b) the net work output,
c) the thermal efficiency, and
(d) the mean effective pressure for the cycle.

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 16:10
An electrical motor raises a 50kg load at a construct velencity .calculate the power of the motor, if it takes 40sec to raise the load through a height of 24m(take g =9.8n/g)
Answers: 2

Engineering, 04.07.2019 18:10
Apump is used to circulate hot water in a home heating system. water enters the well-insulated pump operating at steady state at a rate of 0.42 gal/min. the inlet pressure and temperature are 14.7 lbf/in.2, and 180°f, respectively; at the exit the pressure is 60 lbf/in.2 the pump requires 1/15 hp of power input. water can be modeled as an incompressible substance with constant density of 60.58 lb/ft3 and constant specific heat of 1 btu/lb or. neglecting kinetic and potential energy effects, determine the temperature change, in °r, as the water flows through the pump.
Answers: 1

Engineering, 04.07.2019 18:10
During a steady flow process, the change of energy with respect to time is zero. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10
The filament of an incandescent lamp has a temperature of 2000k. calculate the fraction of radiation emitted in the visible light band if the filament is approximated as blackbody
Answers: 2
You know the right answer?
An ideal otto cycle has a compression ratio of 8. at the begining of the compression process, air is...
Questions




Mathematics, 10.10.2020 22:01

Mathematics, 10.10.2020 22:01







Mathematics, 10.10.2020 22:01

English, 10.10.2020 22:01

Mathematics, 10.10.2020 22:01

Arts, 10.10.2020 22:01

History, 10.10.2020 22:01

Health, 10.10.2020 22:01

English, 10.10.2020 22:01

Geography, 10.10.2020 22:01

Mathematics, 10.10.2020 22:01

![T_2=T_1[\frac{V_1}{V_2}]^{K-1}](/tpl/images/0504/2595/bb0fa.png)
![T_2=300[8]^{1.4-1} = 300[8]^{0.4} = 689.22K](/tpl/images/0504/2595/80b7b.png)
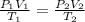
![P_2 =P_1[\frac{T_2}{T_1}][\frac{V_1}{V_2}] = 95 Kpa[\frac{689.22}{300}](8)](/tpl/images/0504/2595/e6217.png)
![P_3 =P_2[\frac{T_3}{T_2}][\frac{V_2}{V_3}] = 1746.024 Kpa[\frac{1733.79}{689.22}](1)](/tpl/images/0504/2595/eec37.png)
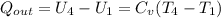
![T_4=T_3[\frac{V_3}{V_4}]^{K-1} = 1733.79 [\frac{1}{8}]^{0.4} = 754.68K](/tpl/images/0504/2595/9e059.png)
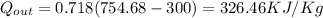
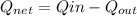 = (750 - 326.46) KJ/Kg = 423.54 KJ/Kg
= (750 - 326.46) KJ/Kg = 423.54 KJ/Kg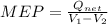 =
=