Engineering, 13.11.2019 06:31 carnations
Aprocess consists of two steps: (1) one mole of air at t = 800 k and p = 4 bar is cooled at constant volume to t = 350 k. (2) the air is then heated at constant pressure until its temperature reaches 800 k. if this two-step process is replaced by a single isothermal expansion of the air from 800 k and 4 bar to some final pressure p, what is the value of p that makes the work of the two processes the same? assume mechanical reversibility and treat air as an ideal gas with cp = (7/2)r and cv = (5/2)r.

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 15:10
If you were designing a bumper for a car, would you prefer it to exhibit elastic or plastic deformation? why? consider the functions of a bumper in both a minor "fender-bender" and a major collision.
Answers: 1

Engineering, 04.07.2019 18:10
Different types of steels contain different elements that alter the characteristics of the steel. for each of the following elements, explain what the element does when alloyed with steel.
Answers: 2

Engineering, 04.07.2019 18:10
Carbon dioxide gas expands isotherm a turbine from 1 mpa, 500 k at 200 kpa. assuming the ideal gas model and neglecting the kinetic and potential energies, determine the change in entropy, heat transfer and work for each kilogram of co2.
Answers: 2

Engineering, 04.07.2019 18:20
Acertain flow of air (at stp) has a velocity distribution given by v i (in ft/s). if this flow is going through a 4 ft square area in the yz-plane (centered at the origin), what is the mass flow rate (in lbm/s)?
Answers: 2
You know the right answer?
Aprocess consists of two steps: (1) one mole of air at t = 800 k and p = 4 bar is cooled at constan...
Questions




Computers and Technology, 27.08.2019 20:30

English, 27.08.2019 20:30


Geography, 27.08.2019 20:30


Mathematics, 27.08.2019 20:30

History, 27.08.2019 20:30

Mathematics, 27.08.2019 20:30

Computers and Technology, 27.08.2019 20:30

Geography, 27.08.2019 20:30

Chemistry, 27.08.2019 20:30


English, 27.08.2019 20:30

History, 27.08.2019 20:30

Health, 27.08.2019 20:30

Mathematics, 27.08.2019 20:30

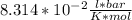 here.
here. = 0.1 kJ
= 0.1 kJ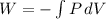 eq1
eq1 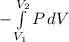
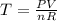
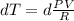

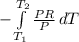
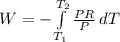
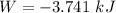
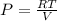
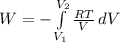
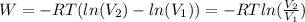 eq2
eq2 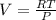
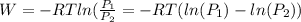
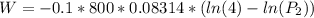
 of process 2 that would make that work done by both process equal. So, we equate the work done by both process
of process 2 that would make that work done by both process equal. So, we equate the work done by both process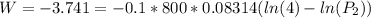
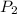 and we get
and we get