
Engineering, 19.10.2019 05:30 hamidaakter936848
An fcc iron–carbon alloy initially containing 0.35 wt% c is exposed to an oxygen-rich and virtually carbon-free atmosphere at 1400 k (1127 °c). under these circumstances the carbon diffuses from the alloy and reacts at the surface with the oxygen in the atmosphere; that is, the carbon concentration at the surface position is maintained essentially at 0 wt% c. (this process of carbon depletion is termed decarburization.) at what position will the carbon concentration be 0.15 wt% after a 10-h treatment? the value of d at 1400 k is 6.9 x 10-11 m2 /s. g

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 18:10
Afluid flows with a velocity field given by v=(x/t)i.. determine the local and convective accelerations when x=3 and t=1.
Answers: 2

Engineering, 04.07.2019 18:10
The higher the astm grain-size number, the coarser the grain is. a)-true b)-false
Answers: 3

Engineering, 04.07.2019 18:10
Ajournal bearing has a journal diameter of 3.250 in with a unilateral tolerance of 20.003 in. the bushing bore has a diameter of 3.256 in and a unilateral tolerance of 0.004 in. the bushing is 2.8 in long and supports a 700-lbf load. the journal speed is 900 rev/min. find the minimum oil film thickness and the maximum film pressure for both sae 20 and sae 20w-30 lubricants, for the tightest assembly if the operating film temperature is 160°f. a computer code is appropriate for solving this problem.
Answers: 3

Engineering, 04.07.2019 18:10
Courses that are developed by subject matter experts, internal or extemal to the college or university. these programs are marketed by the school (clo2) marks a)-vocational schools b)-vendor training c)-colleges & universities d)-continuing education programs
Answers: 2
You know the right answer?
An fcc iron–carbon alloy initially containing 0.35 wt% c is exposed to an oxygen-rich and virtually...
Questions

Computers and Technology, 03.01.2020 23:31




Mathematics, 03.01.2020 23:31

Social Studies, 03.01.2020 23:31

History, 03.01.2020 23:31

Mathematics, 03.01.2020 23:31

English, 03.01.2020 23:31

English, 03.01.2020 23:31




History, 03.01.2020 23:31

History, 03.01.2020 23:31





Mathematics, 03.01.2020 23:31


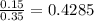
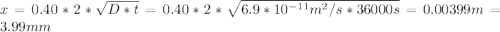 t} )[/tex]
t} )[/tex]

