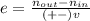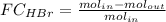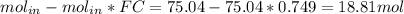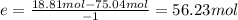Engineering, 16.10.2019 05:00 jnsoccerboy3121
The reaction between ethylene and hydrogen bromide to form ethyl bromide is carried out in a continuous reactor. the product stream is analyzed and found to contain 51.7 mole% c2h5br and 17.3% hbr. the feed to the reactor contains only ethylene and hydrogen bromide. calculate the fractional conversion of the limiting reactant and the percentage by which the other reactant is in excess. if the molar flow rate of the feed stream is 165mol/s, what is the extent of reaction?

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 18:10
The temperature of air decreases as it is compressed by an adiabatic compressor. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10
During a steady flow process, the change of energy with respect to time is zero. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10
Thermal stresses are developed in a metal when its a) initial temperature is changed b) final temperature is changed c) density is changed d) thermal deformation is prevented e) expansion is prevented f) contraction is prevented
Answers: 2

Engineering, 04.07.2019 18:10
At 12 noon, the count in a bacteria culture was 400; at 4: 00 pm the count was 1200 let p(t) denote the bacteria cou population growth law. find: (a) an expression for the bacteria count at any time t (b) the bacteria count at 10 am. (c) the time required for the bacteria count to reach 1800.
Answers: 1
You know the right answer?
The reaction between ethylene and hydrogen bromide to form ethyl bromide is carried out in a continu...
Questions

Mathematics, 06.01.2021 22:10

Mathematics, 06.01.2021 22:10




Mathematics, 06.01.2021 22:10

Social Studies, 06.01.2021 22:10



Mathematics, 06.01.2021 22:10



History, 06.01.2021 22:10

Biology, 06.01.2021 22:10


Mathematics, 06.01.2021 22:10


Chemistry, 06.01.2021 22:10


English, 06.01.2021 22:10


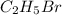 = 51.7%
= 51.7%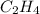 = 100% - 69% = 31%
= 100% - 69% = 31%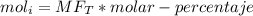
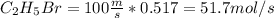
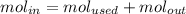
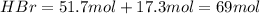
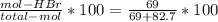 = 45.4%
= 45.4%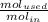
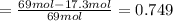
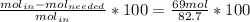 =16.56%
=16.56%