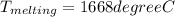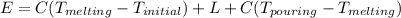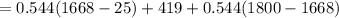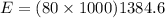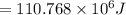Engineering, 11.10.2019 04:20 JustinLaucirica
The melting temperature of pure titanium = 1668°c, its density = 4.5 g/cm^3, specific heat = 0.544 j/gºc, and heat of fusion = 419 j/g. assume specific heat has the same value for solid and molten metal. the pouring temperature for titanium is 1800°c, and the starting temperature = 25°c. estimate the (a) energy for heating unit mass (1 g pure titanium) to pouring temperature and (b) total energy to heat 80 kg of the metal.

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 18:10
The temperature of air decreases as it is compressed by an adiabatic compressor. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:20
Describe one experiment in which the glass transition temperature and melting temperature of a totally amorphous thermoplastic material can be determined. show the relevant experimental results in a diagram which should be properly annotated with the two temperatures clearly marked. what is likely to happen to the curve in the diagram if the amorphous polymer is replaced by a thermosetting type?
Answers: 2

Engineering, 04.07.2019 18:20
Find the minimum film thickness for a journal bearing with the data below. shaft diameter, d-50 mm, clearance ratio, cdratio? 0.001, shaft speed, n 2000 rpm; bearing length. i 200 mm; eccentricity ration, ? -0.55. ( note, cdratio-ca/d) the minimum film thickness is um
Answers: 2

Engineering, 04.07.2019 18:20
Wiy doeres rere okhn a pump whon working betwon the same pressure range?
Answers: 2
You know the right answer?
The melting temperature of pure titanium = 1668°c, its density = 4.5 g/cm^3, specific heat = 0.544 j...
Questions




Biology, 22.05.2020 17:57


History, 22.05.2020 17:57

History, 22.05.2020 17:57

Mathematics, 22.05.2020 17:57

Mathematics, 22.05.2020 17:57

History, 22.05.2020 17:57


Law, 22.05.2020 17:57

Mathematics, 22.05.2020 17:57

Geography, 22.05.2020 17:57



Physics, 22.05.2020 17:57



Mathematics, 22.05.2020 17:57