
Engineering, 29.09.2019 02:30 daii128
Consider the expansion of a gas at a constant temperature in a water-cooled piston-cylinder system. the constant temperature is achieved by controlled input of energy as heat q to the gas. treating the gas as idea, derive expressions for the energy output as work, w and energy input as heat, q, as a function of the expansion ratio. (v2/v1)

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 15:10
If you were designing a bumper for a car, would you prefer it to exhibit elastic or plastic deformation? why? consider the functions of a bumper in both a minor "fender-bender" and a major collision.
Answers: 1

Engineering, 04.07.2019 12:10
On a average work day more than work place firs are reorted
Answers: 1

Engineering, 04.07.2019 18:10
Determine whether or not it is possible to compress air adiabatically from k to 140 kpa and 400 k. what is the entropy change during this process?
Answers: 3

Engineering, 04.07.2019 18:10
Water in a partially filled large tank is to be supplied to the roof top, which is 8 m above the water level in the tank, through a 2.2-cm-internal-diameter pipe by maintaining a constant air pressure of 300 kpa (gage) in the tank. if the head loss in the piping is 2 m of water, determine the discharge rate of the supply of water to the roof top in liters per second.
Answers: 3
You know the right answer?
Consider the expansion of a gas at a constant temperature in a water-cooled piston-cylinder system....
Questions

World Languages, 27.10.2020 06:00



Geography, 27.10.2020 06:00

History, 27.10.2020 06:00



Business, 27.10.2020 06:00


Biology, 27.10.2020 06:00

Mathematics, 27.10.2020 06:00

Mathematics, 27.10.2020 06:00


Biology, 27.10.2020 06:10

English, 27.10.2020 06:10

Chemistry, 27.10.2020 06:10



Chemistry, 27.10.2020 06:10

Mathematics, 27.10.2020 06:10

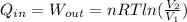
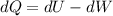
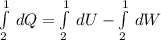
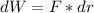
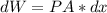
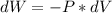
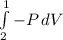
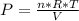 , so:
, so: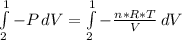
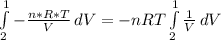
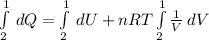
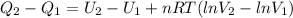
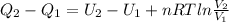
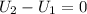 , so the heat exchanged to the system equals the work done by the system:
, so the heat exchanged to the system equals the work done by the system:

