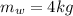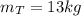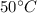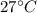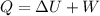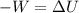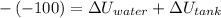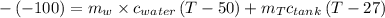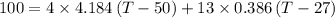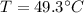Engineering, 24.08.2019 03:30 phancharamachasm
Awell-insulated copper tank of mass 13 kg contains 4 kg of liquid water. initially, the temperature of the copper is 27°c and the temperature of the water is 50°c. an electrical resistor of negligible mass transfers 100 kj of energy to the contents of the tank. the tank and its contents come to equilibrium.

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 14:10
Explain the difference laminar and turbulent flow. explain it with the shear stress and the velocity profiles.
Answers: 1

Engineering, 03.07.2019 15:10
Two flowing streams of argon gas are adiabatically mixed to form a single flow/stream. one stream is 1.5 kg/s at 400 kpa and 200 c while the second stream is 2kg/s at 500 kpa and 100 ? . it is stated that the exit state of the mixed single flow of argon gas is 150 c and 300 kpa. assuming there is no work output or input during the mixing process, does this process violate either the first or the second law or both? explain and state all your assumptions.
Answers: 1

Engineering, 04.07.2019 18:10
Ajournal bearing has a journal diameter of 3.250 in with a unilateral tolerance of 20.003 in. the bushing bore has a diameter of 3.256 in and a unilateral tolerance of 0.004 in. the bushing is 2.8 in long and supports a 700-lbf load. the journal speed is 900 rev/min. find the minimum oil film thickness and the maximum film pressure for both sae 20 and sae 20w-30 lubricants, for the tightest assembly if the operating film temperature is 160°f. a computer code is appropriate for solving this problem.
Answers: 3

Engineering, 04.07.2019 18:10
Carbon dioxide gas expands isotherm a turbine from 1 mpa, 500 k at 200 kpa. assuming the ideal gas model and neglecting the kinetic and potential energies, determine the change in entropy, heat transfer and work for each kilogram of co2.
Answers: 2
You know the right answer?
Awell-insulated copper tank of mass 13 kg contains 4 kg of liquid water. initially, the temperature...
Questions