Engineering, 19.07.2019 23:10 trent1002brown
Determine the total energy e of water vapor with a mass of 2.50 kg, a specific internal energy μ= 2780 kj/kg, traveling at a velocity of 56.0 m/s at a height of 3.50 m. first draw a sketch of your system

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 18:10
Give heat transfer applications for the following, (i) gas turbines (propulsion) ) gas turbines (power generation). (iii) steam turbines. (iv) combined heat and power (chp). (v) automotive engines
Answers: 1

Engineering, 04.07.2019 18:20
Air is compressed isentropically from an initial state of 300 k and 101 kpa to a final temperature of 1000 k. determine the final pressure using the following approaches: (a) approximate analysis (using properties at the average temperature) (b) exact analysis
Answers: 1

Engineering, 04.07.2019 19:10
What are the major differences between injection molding and extrusion?
Answers: 2

Engineering, 06.07.2019 05:20
Amass of 3 kg of saturated liquid-vapor mixture of water is contained in a piston-cylinder device at 150 kpa. initially, 1 kg of the water is in the liquid phase and the rest is in the vapor phase. heat is now transferred to the water, and the piston, which is resting on a set of stops, starts moving when the pressure inside reaches 500 kpa. heat transfer continues until the total volume increases by 20 percent. determine (a)-the initial and final temperatures, (b)-the mass of liquid water when the piston first starts moving, and (c)-the work done during this process. d)-show the process on a p-v diagram. you don't need to indicate p& v values, but clearly indicate where all states are located in the phase diagram.
Answers: 3
You know the right answer?
Determine the total energy e of water vapor with a mass of 2.50 kg, a specific internal energy μ= 27...
Questions

Mathematics, 02.03.2021 21:30


History, 02.03.2021 21:30

Mathematics, 02.03.2021 21:30

Mathematics, 02.03.2021 21:30

Social Studies, 02.03.2021 21:30


Mathematics, 02.03.2021 21:30



French, 02.03.2021 21:30

Biology, 02.03.2021 21:30

Mathematics, 02.03.2021 21:30

English, 02.03.2021 21:30

Computers and Technology, 02.03.2021 21:30


History, 02.03.2021 21:30

Chemistry, 02.03.2021 21:30


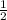 mv² + mgh
mv² + mgh