
Engineering, 19.07.2019 23:10 spalmer8
Determine the theoretical density of a fictitious bcc metal with a gram molecular weight of 148.09 grams per mole and atomic radius of 1.918 angstroms. express result in grams per cubic centimeter. enter numerical values only. do not enter units. do not enter signs.

Answers: 1


Another question on Engineering

Engineering, 03.07.2019 23:20
Two technicians are discussing the intake air temperature (iat) sensor. technician a says that the computer uses the iat sensor as a backup to the engine coolant temperature (ect) sensor. technician b says that the powertrain control module (pcm) will subtract the calculated amount of fuel if the air measures hot. who is correct
Answers: 3

Engineering, 04.07.2019 03:10
What precautions should you take to prevent injuries when dealing with heavy loads?
Answers: 1

Engineering, 04.07.2019 08:10
Which of the following is an easy way to remember the modified “x” tire rotation? a. nondrive wheels straight, cross the drive wheels b. drive wheels straight, cross the nondrive wheels c. drive wheels crossed, nondrive wheels straight d. drive wheels crossed, nondrive wheels crossed
Answers: 1

Engineering, 04.07.2019 18:10
The temperature of air decreases as it is compressed by an adiabatic compressor. a)- true b)- false
Answers: 2
You know the right answer?
Determine the theoretical density of a fictitious bcc metal with a gram molecular weight of 148.09 g...
Questions



Mathematics, 08.01.2020 12:31

Computers and Technology, 08.01.2020 12:31

Mathematics, 08.01.2020 12:31

English, 08.01.2020 12:31


Mathematics, 08.01.2020 12:31

History, 08.01.2020 12:31



SAT, 08.01.2020 12:31

Social Studies, 08.01.2020 12:31

History, 08.01.2020 12:31

Biology, 08.01.2020 12:31



History, 08.01.2020 12:31

History, 08.01.2020 12:31

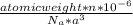
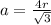
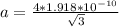
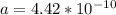
![\frac{148.06 *2*10^{-6}}{6.023 *10^{23}*[4.42 *10^{-10}]^{3}}](/tpl/images/0109/5539/989fb.png)


