Engineering, 19.07.2019 20:30 naomirice24
Agas contained within a piston-cylinder undergoes the follow change in states: process 1: constant volume from p1 = 1 bar v1 = 2.6 m3 to state 2 with p2 = 2.7 bar process 2: compression to v3 = 1.5 m3, which the pressure-volume relationship is pv = constant. process 3: constant pressure to state 4, where v4 = 0.5 m3. sketch the processes on p-v graph and evaluate the work for each process in kj.

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 03:10
What precautions should you take to prevent injuries when dealing with heavy loads?
Answers: 1

Engineering, 04.07.2019 18:20
Find the kinematic pressure of 160kpa. for air, r-287 j/ kg k. and hair al viscosity of air at a temperature of 50°c and an absolute (10 points) (b) find the dynamic viscosity of air at 110 °c. sutherland constant for air is 111k
Answers: 3

Engineering, 04.07.2019 18:20
Aheavily insulated piston-cylinder device contains 0.02 m3 of steam at 300 kpa and 200 °c. 1.2 mpa. d this process. team is now compressed in a reversible manner to a pressure of etermine the entropy change and the work done on the steam during this process
Answers: 1

You know the right answer?
Agas contained within a piston-cylinder undergoes the follow change in states: process 1: constant...
Questions

English, 28.01.2020 18:04

Physics, 28.01.2020 18:04



Mathematics, 28.01.2020 18:04

History, 28.01.2020 18:04


Physics, 28.01.2020 18:05





Mathematics, 28.01.2020 18:05

Social Studies, 28.01.2020 18:05


Mathematics, 28.01.2020 18:05



Mathematics, 28.01.2020 18:05

Mathematics, 28.01.2020 18:05

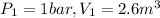
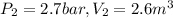
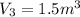
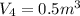
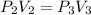
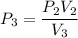
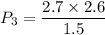
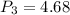 bar
bar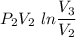
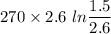 (1 bar=100KPa)
(1 bar=100KPa)