Engineering, 12.07.2019 22:10 Kizmit4938
Apiston-cylinder device contains 1.329 kg of nitrogen gas at 120 kpa and 27 degree c. the gas is now compressed slowly in a polytropic process during which pv^1.49 = constant. the process ends when the volume is reduced by one-half. determine the entropy change of nitrogen during this process.

Answers: 1


Another question on Engineering

Engineering, 03.07.2019 15:10
Apiston-cylinder with a volume of 0.25 m3 holds 1 kg of air (r 0.287 k/kgk) at a temperature of 100 c. heat transfer to the cylinder causes an isothermal expansion of the piston until the volume triples. how much heat is added to the piston-cylinder?
Answers: 3

Engineering, 04.07.2019 18:10
The temperature of air decreases as it is compressed by an adiabatic compressor. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10
The filament of an incandescent lamp has a temperature of 2000k. calculate the fraction of radiation emitted in the visible light band if the filament is approximated as blackbody
Answers: 2

Engineering, 04.07.2019 18:20
Most leaks in reciprocating air compressors can be detected and minimized by: (clo4) a)-detecting leakage areas using ultrasonic acoustic detector. b)-tightening joints and connections c)-replacing faulty equipment d)-all of the given options
Answers: 2
You know the right answer?
Apiston-cylinder device contains 1.329 kg of nitrogen gas at 120 kpa and 27 degree c. the gas is now...
Questions

Chemistry, 24.12.2019 11:31

History, 24.12.2019 11:31


Mathematics, 24.12.2019 11:31



Geography, 24.12.2019 11:31



Mathematics, 24.12.2019 11:31


History, 24.12.2019 11:31

Biology, 24.12.2019 11:31


Mathematics, 24.12.2019 11:31


History, 24.12.2019 11:31

English, 24.12.2019 11:31

Mathematics, 24.12.2019 11:31

History, 24.12.2019 11:31

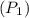 =120KPa
=120KPa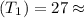 300k
300k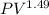 =constant
=constant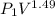 =
=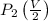
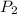 =337.066KPa
=337.066KPa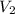 =
=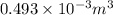
 =
=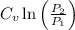 +
+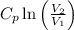
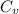 =
=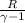 =0.6059
=0.6059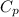 =
=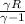 =0.9027
=0.9027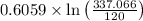 +
+