Chemistry, 21.07.2019 07:00 LeoInc6806
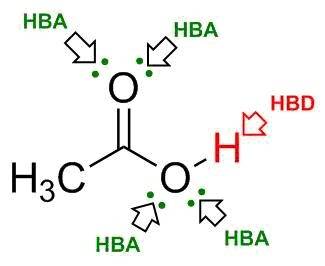
Ch3cooh (acetic acid) can form hydrogen bonds between its molecules. based on the lewis structure shown below, how many hydrogen bond donor and acceptor atoms does this molecule have?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1


Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
Ch3cooh (acetic acid) can form hydrogen bonds between its molecules. based on the lewis structure sh...
Questions

Mathematics, 27.08.2021 04:40



Mathematics, 27.08.2021 04:40


Mathematics, 27.08.2021 04:40







Mathematics, 27.08.2021 04:40


History, 27.08.2021 04:40

English, 27.08.2021 04:40


History, 27.08.2021 04:40