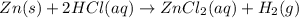Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3

Chemistry, 23.06.2019 19:00
What is the final temperature after 840 joules is absorbed by 10.0g of water at 25.0c
Answers: 1
You know the right answer?
Given the reaction: zn(s) + 2hcl(aq) → zncl2(aq) + h2(g) the oxidation number of zn(s) increases be...
Questions

Mathematics, 28.01.2020 08:31

Mathematics, 28.01.2020 08:31


Geography, 28.01.2020 08:31

Geography, 28.01.2020 08:31

Computers and Technology, 28.01.2020 08:31




Mathematics, 28.01.2020 08:31

Mathematics, 28.01.2020 08:31





History, 28.01.2020 08:31


Biology, 28.01.2020 08:31

History, 28.01.2020 08:31

Mathematics, 28.01.2020 08:31