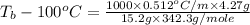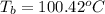Chemistry, 21.07.2019 11:00 joanasprinkman2262
If 4.27 g sucrose (c12h22o11) are dissolved in 15.2 g water, what is the boiling point of the resulting solution? kb for water = 0.512c/m.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2


Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
If 4.27 g sucrose (c12h22o11) are dissolved in 15.2 g water, what is the boiling point of the result...
Questions


Mathematics, 27.10.2020 19:40


Mathematics, 27.10.2020 19:40

History, 27.10.2020 19:40

Chemistry, 27.10.2020 19:40

Mathematics, 27.10.2020 19:40




Mathematics, 27.10.2020 19:40

Mathematics, 27.10.2020 19:40

Mathematics, 27.10.2020 19:40

Health, 27.10.2020 19:40

Arts, 27.10.2020 19:40



Chemistry, 27.10.2020 19:40


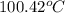
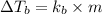
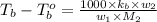
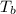 = boiling point of solution = ?
= boiling point of solution = ?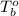 = boiling point of pure water =
= boiling point of pure water = 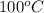
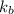 = boiling point constant for water =
= boiling point constant for water = 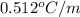
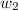 = mass of solute (sucrose) = 4.27 g
= mass of solute (sucrose) = 4.27 g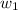 = mass of solvent (water) = 15.2 g
= mass of solvent (water) = 15.2 g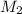 = molar mass of solute (sucrose) = 342.3 g/mole
= molar mass of solute (sucrose) = 342.3 g/mole