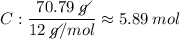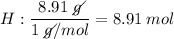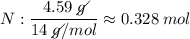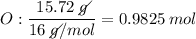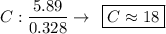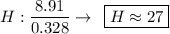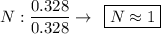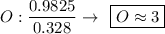Determine the empirical formula for a compound that is 70.79% carbon, 8.91% hydrogen, 4.59% nitrogen, and 15.72% oxygen. determine the empirical formula for a compound that is 70.79 carbon, 8.91 hydrogen, 4.59 nitrogen, and 15.72 oxygen. c18h27no2 c18h27no3 c17h27no3 c17h26no3

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

You know the right answer?
Determine the empirical formula for a compound that is 70.79% carbon, 8.91% hydrogen, 4.59% nitrogen...
Questions

Mathematics, 01.02.2020 22:43

History, 01.02.2020 22:43






Mathematics, 01.02.2020 22:43


Mathematics, 01.02.2020 22:43


Mathematics, 01.02.2020 22:43

Mathematics, 01.02.2020 22:43


Biology, 01.02.2020 22:43

Mathematics, 01.02.2020 22:43

Physics, 01.02.2020 22:43

Biology, 01.02.2020 22:43

History, 01.02.2020 22:43