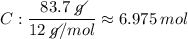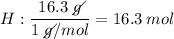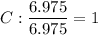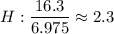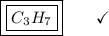Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
What is the empirical formula for a compound that is 83.7% carbon and 16.3% hydrogen?...
Questions



Mathematics, 14.07.2020 21:01


Mathematics, 14.07.2020 21:01

Mathematics, 14.07.2020 21:01

History, 14.07.2020 21:01

Mathematics, 14.07.2020 21:01

English, 14.07.2020 21:01






Mathematics, 14.07.2020 21:01

Biology, 14.07.2020 21:01

Advanced Placement (AP), 14.07.2020 21:01



English, 14.07.2020 21:01