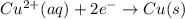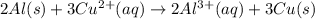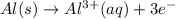Chemistry, 21.07.2019 18:30 theeflyguy5
Given the balanced ionic equation representing a reaction: 2al(s) + 3cu2+(aq) → 2al3+(aq) + 3cu(s)which half-reaction represents the reduction that occurs? al → al3+ +3eal3+ +3e → alcu→ cu2+ +2ecu2+ + 2e → cu

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2


Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

You know the right answer?
Given the balanced ionic equation representing a reaction: 2al(s) + 3cu2+(aq) → 2al3+(aq) + 3cu(s)w...
Questions


History, 17.05.2021 22:20


Mathematics, 17.05.2021 22:20

Mathematics, 17.05.2021 22:20

Mathematics, 17.05.2021 22:20





Biology, 17.05.2021 22:20

SAT, 17.05.2021 22:20

Mathematics, 17.05.2021 22:20


Chemistry, 17.05.2021 22:20

Mathematics, 17.05.2021 22:20




Spanish, 17.05.2021 22:20