

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3


Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 05:30
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3
You know the right answer?
A135 g sample of carbon disulfide requires 43.2 kj of heat to vaporize completely. what is the entha...
Questions

History, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00



History, 27.01.2021 01:00

History, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00

Chemistry, 27.01.2021 01:00

Chemistry, 27.01.2021 01:00






History, 27.01.2021 01:00

Social Studies, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00

Mathematics, 27.01.2021 01:00


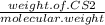
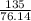
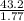 = 24.4 kj/mol
= 24.4 kj/mol

