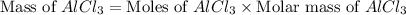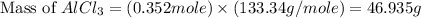Chemistry, 21.07.2019 23:00 hdhdhd49jdhd
If 72.0 grams of aluminum are reacted with 252 grams of hydrochloric acid, how many grams of aluminum chloride are produced by this reaction? 2al(s) + 6hcl(aq) → 2alcl3(aq) + 3h2(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Actual ingredients of lab (the cookies i am actually making) 1/2 cup sugar 1/2 cup brown sugar 1 1/3 stick margarine 1 egg 1/2 tsp salt 1 tsp vanilla 1/2 tsp baking soda 1 1/2 cup flour 1 1/3 cup chocolate chip can you answer the questions below ? discussion 1. suppose you are given the following amounts of ingredients: 1 dozen eggs 24 tsp. of vanilla 1 lb. (82 tsp.) of salt 1 lb. (84 tsp.) of baking soda 3 cups of chocolate chips 5 lb. (11 cups) of sugar 2 lb. (4 cups) of brown sugar 1 lb. (4 sticks) of margarine a. for each ingredient, calculate how many cookies could be prepared if all of that ingredient were consumed. (for example, the recipe shows that using 1 egg- with the right amounts of the other ingredients- yields 24 cookies. how many cookies can you make if the recipe is increased proportionately for 12 eggs? ) b. to determine the limiting reactant for the new ingredients list, identify which ingredient will result in the fewest number of cookies. c. what is the maximum number of cookies that can be produced from the new amounts of ingredients?
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 23.06.2019 06:00
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2
You know the right answer?
If 72.0 grams of aluminum are reacted with 252 grams of hydrochloric acid, how many grams of aluminu...
Questions

Computers and Technology, 27.10.2021 20:40



Physics, 27.10.2021 20:40

Health, 27.10.2021 20:40

Medicine, 27.10.2021 20:40



Mathematics, 27.10.2021 20:40

Mathematics, 27.10.2021 20:40


Mathematics, 27.10.2021 20:40

English, 27.10.2021 20:40

Advanced Placement (AP), 27.10.2021 20:40


English, 27.10.2021 20:40


Physics, 27.10.2021 20:40

English, 27.10.2021 20:40

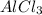 produced will be, 46.935 grams.
produced will be, 46.935 grams.
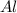 = 9.5 g
= 9.5 g
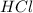 = 130 g
= 130 g
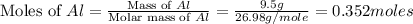
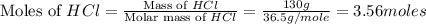
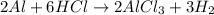
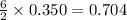 moles of
moles of