
Chemistry, 21.07.2019 23:00 dajeourcrazy15
A5.018 gram sample of a certain hydrate of magnesium sulfate, mgso4•xh2o, is heated until all the water is driven off. the resulting anhydrous compound weighs 2.449 grams. what is the formula of the hydrate?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
A5.018 gram sample of a certain hydrate of magnesium sulfate, mgso4•xh2o, is heated until all the wa...
Questions


Mathematics, 01.06.2021 21:20

Mathematics, 01.06.2021 21:20


Mathematics, 01.06.2021 21:20





Mathematics, 01.06.2021 21:20



Mathematics, 01.06.2021 21:30



Chemistry, 01.06.2021 21:30




Mathematics, 01.06.2021 21:30

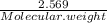
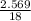
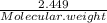
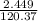
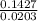 = 7.02 ~7
= 7.02 ~7

