
Chemistry, 22.07.2019 02:01 neparidzetazo
How many molecules of dinitrogen pentoxide are in 1.39 moles of dinitrogen pentoxide

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
How many molecules of dinitrogen pentoxide are in 1.39 moles of dinitrogen pentoxide...
Questions




Mathematics, 03.02.2021 19:30




Mathematics, 03.02.2021 19:30

Mathematics, 03.02.2021 19:30


Biology, 03.02.2021 19:30

Physics, 03.02.2021 19:30

Mathematics, 03.02.2021 19:30


Physics, 03.02.2021 19:30




Mathematics, 03.02.2021 19:30

Mathematics, 03.02.2021 19:30

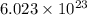 atoms present in 1 mole of a substance.
atoms present in 1 mole of a substance.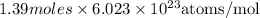
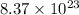 atoms
atoms

