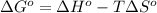Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 21.06.2019 19:10
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

You know the right answer?
Acetylene, c2h2, has a standard enthalpy of formation, δh° = 226.7 kj/mol, and a standard entropy ch...
Questions


Social Studies, 24.06.2019 17:30


English, 24.06.2019 17:30




Mathematics, 24.06.2019 17:30




Computers and Technology, 24.06.2019 17:30

Biology, 24.06.2019 17:30

Chemistry, 24.06.2019 17:30





History, 24.06.2019 17:30

English, 24.06.2019 17:30

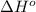 = 226.7 kJ/K mol,
= 226.7 kJ/K mol, 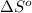 = 58.8 J/mol =
= 58.8 J/mol = 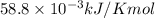
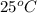 = (25 + 273) K = 298 K
= (25 + 273) K = 298 K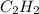 ) as follows.
) as follows.