
Chemistry, 22.07.2019 15:00 katelynnjoyce1
Hydrogen gas reacts with oxygen to form water. 2h2( g.+o2( g.→2h2o( g.δh=−483.5kj determine the minimum mass of hydrogen gas required to produce 216 kj of heat.

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 18:10
Which property of a substance can be determined using a ph indicat
Answers: 3

Chemistry, 23.06.2019 20:30
If 4.88 grams of zn react with 5.03 grams of s8 to produce 6.02 grams of zns, what are the theoretical yield and percent yield of this reaction? be sure to show the work that you did to solve this problem.unbalanced equation: zn + s8 yields zns
Answers: 3

Chemistry, 23.06.2019 22:30
Acompound consisting of b, n, and h undergoes elemental analysis. the % composition by mass is found to be 40.28% b, 52.20% n, and 7.53% h. what is the empirical formula for this molecule? enter in the empirical formula in the form bxnyhz
Answers: 1
You know the right answer?
Hydrogen gas reacts with oxygen to form water. 2h2( g.+o2( g.→2h2o( g.δh=−483.5kj determine the mini...
Questions

Biology, 03.12.2019 11:31

Mathematics, 03.12.2019 11:31

Mathematics, 03.12.2019 11:31


Mathematics, 03.12.2019 11:31



English, 03.12.2019 11:31

Mathematics, 03.12.2019 12:31

Mathematics, 03.12.2019 12:31

Mathematics, 03.12.2019 12:31


Mathematics, 03.12.2019 12:31


Mathematics, 03.12.2019 12:31


Mathematics, 03.12.2019 12:31

Mathematics, 03.12.2019 12:31


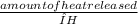 x 2 mol H₂
x 2 mol H₂


